How Dry Cell Batteries Generate Electricity
Normally a dry cell is also
referred to as a Zinc-Carbon Leclanche cell. It is an easily portable, compact,
and modified form of Leclanche cell capable of producing an EMF of 1.5 V with a
very small internal resistance in the order of 0.1 ohm.
Dry Cell History and Advances
The first dry cell was
invented in the late 19th century. It used zinc as an anode, manganese dioxide
as an “earthode," and a gelled, moist mixture of ammonium chloride and
zinc chloride as electrolyte.
Later they created a dry cell
made up of carbon as a cathode, zinc as an anode, and sal-ammoniac paste as an
electrolyte. This type of dry cell is commonly known as a carbon zinc Leclanche
cell. Even today, most of the dry cells manufactured are of this kind due to
its lower manufacturing cost and its being suitable for all applications
requiring intermittent current, such as used in flashlights and transistor
receivers.
These cells have a few
drawbacks such as their low energy density and limited lifetime. In later
years, a large number of new types of dry cells were developed for new and
different applications.
Modification in Leclanche
Cell to become a Dry Cell
The glass in Leclanche cell is
replaced by a zinc container, and the ammonium chloride solution is replaced by
a moist sal-ammoniac paste.
The Dry Cell is a Primary
Cell
The cells from which electric
energy is derived by irreversible chemical action are called primary cells. The
primary cell is capable of providing an EMF when its constituent’s two
electrodes and a suitable electrolyte are assembled together. The three main
primary cells namely are the Daniel cell, the Leclanche cell, and the dry cell.
None of these cells can be recharged electrically.
Commercial Dry Cells


How Chemical Energy is converted into Electrical Energy in Cells
Chemical effect of current
Conversion of electric energy
into chemical energy: the passage of an electric current through a liquid
causes chemical changes through a process called electrolysis. Conduction is
possible only in liquids wherein charged ions can be dissociated in opposite
directions. Such liquids are called electrolytes, and the plates through which
current enters and leaves an electrolyte are known as electrodes. The electrode
towards which positive ions travel is called the cathode, and the electrode
towards which negative ions travel is called the anode. The positive ions are
called cations and negative ions are called anions.
Effect of Chemicals in
Batteries
Conversion of chemical energy
into electrical energy: in this case, the reverse process takes place due to
the chemical reaction between two electrodes in the presence of an electrolyte
and an electric current is produced.
Faraday’s Laws of Electrolysis
First law:
The mass of a substance
liberated at an electrode is directly proportional to the charge passing
through the electrode.
Second law:
The mass of a substance liberated
at an electrode by a given amount of charges is proportional to the chemical
equivalent of the substance.
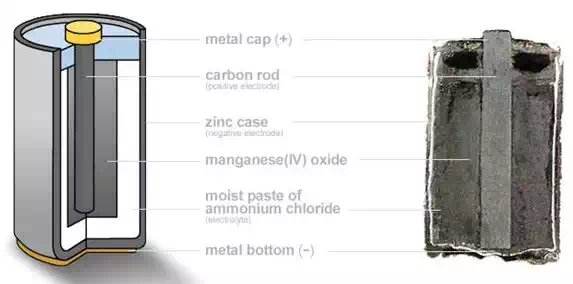
Working of a Dry Cell:
Parts:
Anode (Negative
Terminal): Zinc
Cathode (Positive
Terminal): Carbon
coated with MnO2
Electrolyte: Mixture of plaster of Paris, Ammonium
Chloride and Zinc Chloride
Dry cells contain a Zinc
container which itself acts as a negative electrode. The moist paste is made
from a mixture of plaster of Paris, Ammonium Chloride, and Zinc Chloride called
sal ammoniac paste. This forms the electrolyte of the cell and takes up the
major amount of volume in the battery. Zinc Chloride is hygroscopic in nature
and helps to maintain the moistness of the paste. It is wrapped in a canvas
sheet.
○ Anode reaction: The oxidization
of Zinc gives two electrons.
Zn(solid) →
Zn2 + (aqueous) + 2 (e-)
The carbon rod forms the
positive electrode. It is coated with MnO2 and powdered carbon. The powdered
carbon reduces the internal resistance of the cell. The top of the cell contains
a layer of sawdust. This acts as the base for the top layer of bitumen used for
sealing purposes.
○ Cathode reaction:
2MnO2(solid) + H2(gas)→
Mn2O3(solid) + H2O(liquid)
○ Electrolyte reaction: Hydrogen
from Ammonium chloride
2NH4 + (aqueous )
+ 2 (e-) → H2(g) + 2NH3(aqueous)
○ Overall reaction in dry cell:
Zn(s) + 2MnO2(s)
+ 2NH4(+)(aqueous) → Mn2O3(solid) + Zn(NH3)2 (2+)(aqueous)
+ H2O(liquid)
A vent is provided in this
layer to allow the gases formed in the chemical reaction to escape.
Irrespective of the size of the dry cell, the EMF is 1.5 V because the zinc and
carbon rods used as electrodes specified a chemical equivalent. The chemical
equivalent changes from metal to metal and, depending on the type of
combination used, the EMF differs.
Dry Cell Parts