Battery
Technology
In the
modern era, electrical energy is normally converted from mechanical energy,
solar energy, and chemical energy etc. A battery is a device
that converts chemical energy to electrical energy. The first battery was
developed by Alessandro Volta in the year of 1800. In the year 1836, John
Frederic Daniell, a British chemist developed
the Daniell cell as an improved version of
the voltaic cell. From that time until
today, the battery has been the most popular source of
electricity in many daily life applications.In our
daily life, we generally use two types of battery, one of them is which can be
used once before it gets totally discharged. Another type of battery is
rechargeable which means it can be used multiple times by recharging it
externally. The former is called primary battery and the later is called secondary battery.
Batteries can be found in different sizes. A battery may be as small as a shirt
button or may be so big in size that a whole room will be required to install a
battery bank. With this variation of sizes, the battery is used anywhere from
small wrist watches to a large ship.
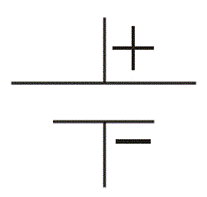
We often see this symbol in many diagrams of electrical and electronics network.
This is the most popularly used symbol for battery. The bigger lines represent
positive terminal of the cells and smaller lines represent negative terminal of
the cells connected in the battery.
We are
often confused about the terms battery cell and battery
. We generally refer a battery as a single electro-chemical cell.
But literally, battery does not mean that. Battery means a number of
electro-chemical cells connected together to meet a certain voltage and current level. Although
there may be a single cell battery, literally, battery and
cell are different.
History of Battery
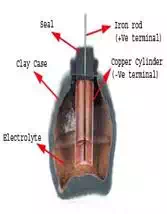 In the year of 1936 during the middle of summer, an ancient tomb
was discovered during construction of a new railway line near Bagdad city in
Iraq. The relics found in that tomb were about 2000 years old. Among these
relics, there were some clay jars or vessels which were sealed at the top with
pitch. An iron rod, surrounded by a cylindrical tube made of wrapped copper
sheet was projected out from this sealed top.
In the year of 1936 during the middle of summer, an ancient tomb
was discovered during construction of a new railway line near Bagdad city in
Iraq. The relics found in that tomb were about 2000 years old. Among these
relics, there were some clay jars or vessels which were sealed at the top with
pitch. An iron rod, surrounded by a cylindrical tube made of wrapped copper
sheet was projected out from this sealed top.
When these pots were filled with an acidic liquid, they produced a potential
difference of around 2 volts between the iron and copper. These clay jars are
suspected to be 2000 year old battery cells.
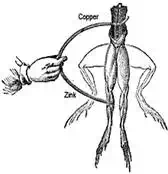
In 1786, Luigi Galvani, an Italian anatomist and physiologist was surprised to
see that when he touched a dead frogís leg with two different metals, the
muscles of the legs contracted. He could not understand the actual reason why,
otherwise he would have been known as the first inventor of the battery cell.
He thought the reaction might be due to a property of the tissues.
After that, Alessandro Volta realized that same phenomenon could be created by
using cardboard soaked in salt water instead of frog's leg. He sandwiched a
copper disc and a zinc disc with a piece of cardboard soaked in salt water in
between them and found a potential difference between the copper and zinc.
After that in 1800, he developed the first Voltaic Pile (battery) constructed
of alternating copper and zinc discs with pieces of cardboard soaked in brine
between them. This system could produce measurable current. Alessandro Volta's
voltaic pile was considered the first "wet battery cell". Thus,
the history of battery began.
The main problem with the Voltaic pile was that, it could not deliver current for a long time.
This problem was solved by a British inventor John F. Daniell in
1836. He invented a more developed version of the battery cell which is known
as the Daniell cell. Here in this cell, one
zinc rod is immersed in zinc sulfate in one
container and one copper rod is immersed in copper (II) sulfate in another container. The solutions of these
two containers are bridged by a U shaped salt bridge. A Daniell cell could produce 1.1 volt and this type of
battery lasted much longer than the Voltaic pile.
In 1839, the fuel cell was designed by Sir William Robert Grove, a discoverer
and man of science. He mixed hydrogen and oxygen within an electrolyte
solution, and created electricity and water. The fuel cell did not deliver
enough electricity, but it is helpful.
Bunsen (1842) and Grove (1839) created enhancements to battery that used liquid
electrodes to supply electricity.

In the year of 1859, Gaston Plante; first
developed the lead acid battery cell.
This was the first form of rechargeable secondary battery. The lead acid battery is
still in use for many industrial purposes. It is still the most popular to be
used as car battery.
In 1866, the battery was again developed by a French engineer, Georges Leclanche. It was a carbon-zinc wet cell battery known as
the Leclanche cell. Crushed manganese
dioxide mixed with a bit of carbon forms the positive electrode and a zinc rod
is used as the negative electrode. Ammonium chloride solution is used as a
liquid electrolyte. After some years, Georges Leclanche himself
improved his own design by replacing liquid ammonium chloride solution with
ammonium chloride. This was the invention of the first dry cell.
In 1901, Thomas Alva Edison discovered the alkaline accumulator. Thomas
Edison's basic cell had iron as the anode material (-) and nickel oxide as the
cathode material(+). This is just one portion of
an endless history of battery .
Step by Step Development in History of Batteries
|
Developer/Inventor |
Country |
Year |
Invention |
|
Luigi
Galvani |
Italy |
1786 |
Animal
Electricity |
|
Alessandro
Volta |
Italy |
1800 |
Voltaic
Pile |
|
John
F. Daniell |
Britain |
1836 |
Daniell Cell |
|
Sir
William Robert Grove |
Britain |
1839 |
Fuel
Cell |
|
Robert
Bunsen |
German |
1842 |
used
liquid electrodes to supply electricity |
|
Gaston Plante |
France |
1859 |
Lead
Acid Battery |
|
Georges Leclanche |
France |
1866 |
Leclanche Cell |
|
Thomas
Alva Edison |
United
States |
1901 |
Alkaline
Accumulator |
Working Principle of Battery
To understand the basic principle of battery properly, first,
we should have some basic concept of electrolytes and electrons affinity.
Actually, when two dissimilar metals or metallic compounds are immersed in an
electrolyte, there will be a potential difference produced between these metals
or metallic compounds.
It is found that, when some specific compounds are added to water, they get
dissolved and produce negative and positive ions. This type of compound is
called an electrolyte. The popular examples of electrolytes are almost all
kinds of salts, acids, and bases etc.
The energy released during accepting an electron by a neutral atom is known as
electron affinity. As the atomic
structure for
different materials are different, the electron affinity of different materials
will differ. If two different kinds of metals or metallic compounds are
immersed in the same electrolyte solution, one of them will gain electrons and
the other will release electrons. Which metal (or metallic compound) will gain
electrons and which will lose them depends upon the electron affinities of
these metals or metallic compounds. The metal with low electron affinity will
gain electrons from the negative ions of the electrolyte solution. On the other
hand, the metal with high electron affinity will release electrons and these
electrons come out into the electrolyte solution and are added to the positive
ions of the solution. In this way, one of these metals or compounds gains
electrons and another one loses electrons. As a result, there will be a
difference in electron concentration between these two metals. This difference
of electron concentration causes an electrical
potential difference to develop between the metals. This electrical potential
difference or emf can be utilized as a source of voltage in any
electronics or electrical
circuit.
This is a general and basic principle of battery .
All
batteries cells are based only on this basic principle. Letís discuss one by
one. As we said earlier, Alessandro Volta developed the first battery cell, and
this cell is popularly known as the simple voltaic
cell.
This type of simple cell can be created very easily. Take one container and
fill it with diluted sulfuric acid as the electrolyte. Now immerse zinc and one
copper rod in the solution and connect them externally by an electric load. Now
your simple voltaic cell is completed. Current will start flowing through the
external load.
Zinc in diluted sulfuric acid gives up electrons as below:
![]()
![]()
These Zn + + ions pass into the electrolyte, and their
concentration is very high near the zinc electrode. As a result of the above
oxidation reaction, the zinc electrode is left negatively charged and hence
acts as cathode. The diluted sulfuric acid and water disassociate into
hydronium ions as given below:
![]()
![]()
Due to the high concentration of Zn + + ions near the
cathode, the H3O+ ions are repelled towards the
copper electrode and get discharged by removing electrons from the copper
atoms. The following reaction takes place at the anode:
![]()
![]()
As a result of the reduction reaction taking place at copper electrode, copper
is left positively charged and hence it acts as the anode.
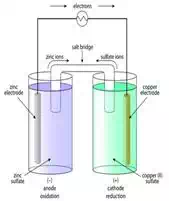
Daniell Battery Cell: The Daniell cell
consists of a copper vessel containing copper sulfate solution.
The copper vessel itself acts as the positive electrode. A porous pot
containing diluted sulfuric acid is placed in the copper vessel. An amalgamated
zinc rod dipping inside the sulfuric acid acts as the negative electrode.
When the circuit is completed, diluted sulfuric acid in the porous pot reacts
with zinc so as to liberate hydrogen gas. The reaction takes place as below:
![]()
![]()
The formation of ZnSO4 in the porous pot does not affect the
working of the cell, until crystals of ZnSO4 are deposited.
The hydrogen gas passes through the porous pot and reacts with the CuSO4 solution
as below:
![]()
![]()
Copper so formed gets deposited on the copper vessel.