Nature of Electricity
Electricity is the most common form of energy. Electricity is used for
various applications such as lighting, transportation, cooking, communication,
production of various goods in factories and much more. None of us exactly know
that what is electricity. The concept
of electricity and theories behind it, can be developed by observing
its different behaviors. For observing nature
of electricity, it is necessary to study the structure of matters. Every
substance in this universe is made up of extremely small particles known as
molecules. The molecule is the smallest particle of a substance into which all
the identities of that substance are present. The molecules are made up of
further smaller particles known as atoms. An atom is the smallest particle of
an element that can exist.
There are two types of substances. The substance, that's molecules are
made of similar atoms is known as an element. The matter whose molecules
consisting dissimilar atoms, is called a compound. The concept of
electricity can be achieved from the atomic structures of
substances.
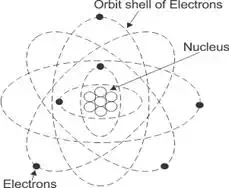
Atoms may have loosely bonded electrons in their outermost orbits. These
electrons require a very small amount of energy to detach themselves from their
parent atoms. These electrons are referred as free electrons which move
randomly inside the substance and transferred from one atom to another. Any
piece of substances which as a whole contains an unequal number of electrons
and protons is referred as electrically charged. When there is more number of
electrons compared to its protons, the substance is said to be negatively
charged and when there is more number of protons compared to electrons, the
substance is said to be positively charged.
The basic nature of electricity is, whenever a
negatively charged body is connected to a positively charged body by means of a
conductor, the excess electrons of negative body starts flowing towards the
positive body to compensate the lack of electrons in that positive body. 
Hope you got the very basic concept of electricity from the
above explanation. There are some materials which have plenty of free electrons
at normal room temperature. Very well known examples
of this type of materials are, silver, copper, aluminium, zinc etc. The
movement of these free electrons can easily be directed to a particular
direction if the electrical potential difference is applied across the piece of
these materials. Because of plenty of free electrons these materials have good
electrical conductivity. These materials are referred as good conductor. The
drift of electrons in a conductor in one direction is known as the current.
Actually electrons flow from lower potential (-Ve) to
higher potential (+Ve) but the general conventional
direction of current has been considered as the highest potential point to
lower potential point, so the conventional direction of current has been just
opposite of the direction of flow of electrons. In non-metallic materials, such
as glass, mica, slate, porcelain, the outermost orbit is completed and there is
almost no chance of loosing electrons from
its outermost shell. Hence there is hardly any free electron present in this
type of material.
Hence, these materials cannot conduct electricity in other words electrical
conductivity of these materials is very poor. Such material are known as non -
conductor or electrical insulator. The nature of electricity is
to flow through a conductor while an electrical potential difference applied
across it, but not to flow through insulator even high electrical potential
difference applied across them.