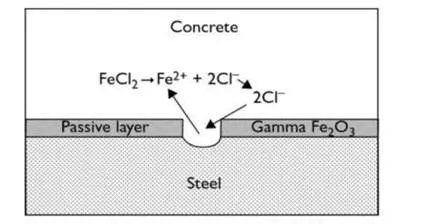
Chloride attack
The chloride ion attacks the passive layer although in this case (unlike carbonation) there is no overall drop in pH. Chlorides act as catalysts to corrosion. They are not consumed in the process but help to break down the passive layer of oxide on the steel and allow the corrosion process to proceed quickly as shown in fig.5. The effective recycling of chloride ions makes chloride attack more difficult to remedy as chlorides are therefore harder to eliminate. Fig.4 shows the recycling of chloride ion. Obviously a few chloride ions in the pore water will not break down the passive layer.