Prevention of corrosion in RCC by bacteria
Abstract
Steel gets
oxidise (corrosion) in the present of oxygen and water. Even present of oxygen
in the concrete pore will not cause a corrosion at high alkaline environment.
Concrete contains microscopic pores which contain high concentrations of
soluble calcium, sodium and potassium oxides, this creates alkaline condition
of pH 12–13. The alkaline condition leads to a ‘passive’ layer forming on the
steel surface. The dense passive layer over the reinforcement prevents the
alkalinity. This paper involves in the prevention of corrosion by maintaining
alkalinity in concrete by using bacteria.
Introduction
Corrosion in concrete is majorly due to carbonisation and
chlorination. When concrete carbonates to the level of the steel rebar, the
normally alkaline environment, which protects steel from corrosion, is replaced
by a neutral environment. Under these conditions the steel is not passive and
rapid corrosion begins. The rate of corrosion due to carbonated concrete cover
is slower than chloride-induced corrosion. Occasionally, a lack of oxygen
surrounding the steel rebar will cause the metal to dissolve, leaving a low pH
liquid.
Carbon-dioxide combines with water to form acid in which reduce
the pH of concrete by consuming the calcium hydroxide which is formed in
hydration process of cement, at low pH corrosion begins. Prevention of
carbonization, prevent the alkalinity in which corrosion do not take place.
Chloride in the pore of concrete involves during the corrosion only, it just
acts as the catalyst in the corrosion process. Presence of chloride in concrete
pore is inert at alkaline condition.
Corrosion
process
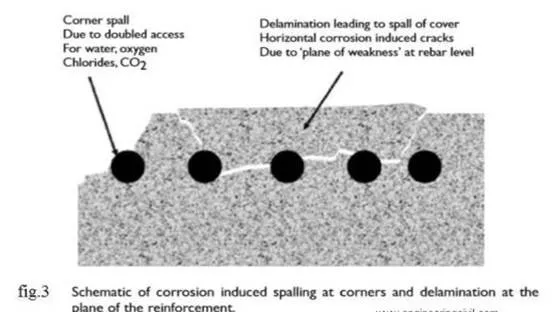
When the passive layer breaks down then rust will start
appearing on the steel surface. The chemical reactions are the same whether
corrosion occurs by chloride attack or carbonation. When steel in concrete
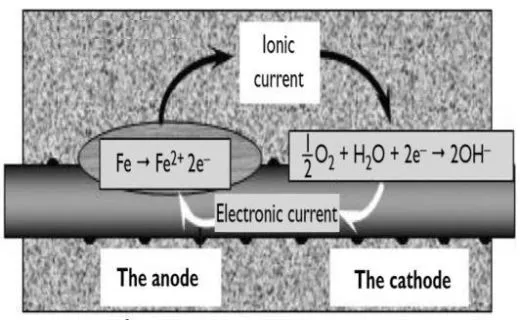
corrodes it dissolves in the pore water and gives up electrons:
The anodic reaction is Fe –> Fe2+ +
2e–
The two electrons (2e) created in the anodic reaction must be
consumed elsewhere on the steel surface to preserve electrical neutrality.
The cathodic reaction is 2e– +
H2O + 1/22 –>2OH–
The ion dissolve in the pore water would not see cracking and
spalling of the concrete. Several more stages must occur for ‘rust’ to form.
Ferrous hydroxide becomes ferric hydroxide and then hydrated ferric oxide or
rust. This rust cause spalling and crack over the concrete as shown in fig.3.
Fig 1. anode and cathode
reaction of corroding bar
Fe2+ and OH– formed in anode and
cathode combined to form ferrous hydroxide and further undergoes chemical
reaction as below
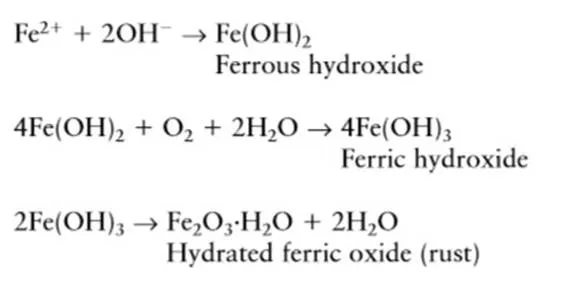
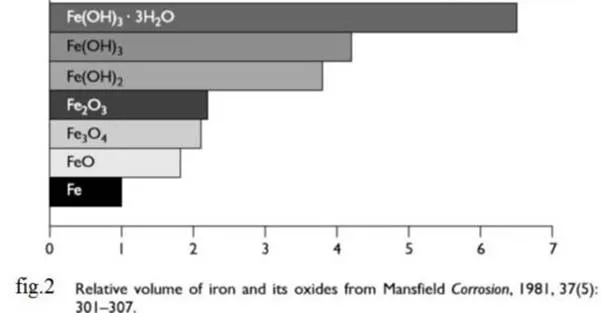
Unhydrated ferric oxide Fe2O3 formed
in the a bow reaction has a volume of about
twice that of the steel it replaces when fully dense. When it becomes hydrated
it swells even more and becomes porous. This means that the volume increase at
the steel/concrete interface is six to ten times . This
leads to the cracking and Spalling as shown in fig.3, that we observe as the
usual consequence of corrosion of steel in concrete and the red/brown brittle,
flaky rust we see on the bar and the rust
stains we see at cracks in the concrete. Fig.2 shows the relative volume of the
iron and its oxide formed in corrosion process.