What Is Phosphine?
Phosphine is a chemical which belongs to the group of organophosphorus compounds. This compound was first obtained by Philippe Gengembre in the year 1783. He obtained phosphine by supplying heat to phosphorous in an aqueous solution of potassium carbonate. Chemical formula of this compound is PH3. The concentration of this compound varies in the atmosphere. It plays a vital role in the phosphorous biochemical cycle.
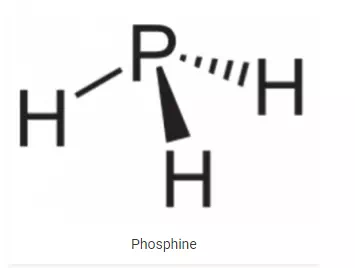
Structure of Phosphine:
PH3 is a trigonal pyramidal molecule. P-H bond length in phosphine is 1.42 A and the angle between H-P-H bonds is 93.5ₒ.
Preparation of Phosphine:
Calcium phosphide is mixed with water or dilutes HCl.This results in the formation of phosphine.
Ca3P2 + 6H2O → 3Ca(OH)2 + 2PH3
Ca3P2 + 6HCl → 3CaCl2 + 2PH3
In the laboratory white phosphorous is heated with concentrated sodium hydroxide solution in an inert atmosphere of CO2 to form phosphine.
P4+3NaOH+3H2O → PH3 + 3NaH2PO2
Physical and chemical properties of phosphine:
· It is a colorless gas having a rotten fish smell.
· It is highly poisonous gas.
· PH3 is sparingly soluble in water and soluble in organic solvents.
· PH3 acts as a Lewis base by donating its lone pair of electrons when it reacts with hydrogen iodide.
· Under normal conditions, it is a non-combustible gas, but when heated it catches fire which results in the formation of phosphoric acid.
· PH3+2O2→150CH3PO4
· When it comes in contact with oxidizing agents it explodes violently.
Uses of phosphine:
In semiconductor industries, it is used in small amounts as a dopant.
PH3 is used in Holme’s signal due to its property of spontaneous combustion.