Continuous distillation columns
In contrast, continuous columns process a continuous feed stream. No interruptions occur unless there is a problem with the column or surrounding process units. They are capable of handling high throughputs. Continuous column is the more common of the two types. Types of Continuous Columns Continuous columns can be further classified according to the nature of the feed that they are processing: Binary distillation column: feed contains only two components Multi-component distillation column: feed contains more than two components the number of product streams they have Multi-product distillation column:
column has more than two product streams where the extra feed exits when it is used to help with the separation, Extractive distillation: where the extra feed appears in the bottom product stream Azeotropic distillation: where the extra feed appears at the top product stream the type of column internals. Tray distillation column: where trays of various designs are used to hold up the liquid to provide better contact between vapor and liquid, hence better separation. The details of the tray column are given in Module 4. Packed distillation column: where instead of trays, 'packings' are used to enhance contact between vapor and liquid. The details of the packed column are given in Module 4.
A single-stage continuous distillation (Flash distillation):
A single-stage continuous operation occurs where a liquid mixture is partially vaporized. The vapor produced and the residual liquids are in equilibrium in the process are separated and removed as shown in Figure 5.8. Consider a binary mixture of A (more volatile component) and B (less volatile component). The feed is preheated before entering the separator. As such, part of the feed may be vaporized. The heated mixture then flows through a pressure-reducing valve to the separator. In the separator, separation between the vapor and liquid takes place. The amount of vaporization affects the concentration (distribution) of A in vapor phase and liquid phase.
The relationship between the scale of vaporization and mole fraction of A in vapor and liquid (y and x) is known as the Operating Line Equation. Define f as molal fraction of the feed that is vaporized and withdrawn continuously as vapor. Therefore, for 1 mole of binary feed mixture, (1- f) is the molal fraction of the feed that leaves continuously as liquid. Assume, yD = mole fraction of A in vapor leaving, xB = mole fraction of A in liquid leaving, xF = mole fraction of A in feed entering. Based on the definition for f, the greater the heating is, the larger the value of f. If the feed is completely vaporized, then f = 1.0 Thus, the value of f can varies from 0 (no vaporization) to 1 (total vaporization). From material balance for the more volatile component (A) one can write

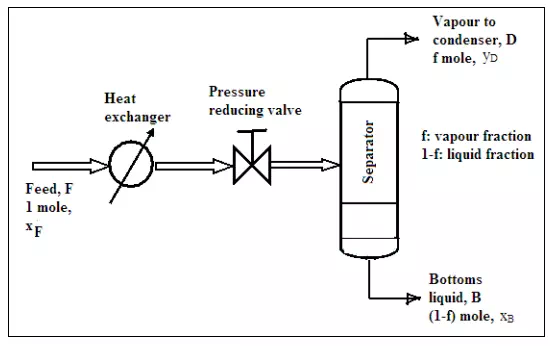

The fraction f depends on the enthalpy of the liquid feed, the enthalpies of the vapor and liquid leaving the separator. For a given feed condition, and hence the known value of f and xF, the Equation (5.12) is a straight line Equation with slope - (1-f)/f and intercept xF/f as shown in Figure 5.9. It will intersect the equilibrium line at the point (xB, yD). From this value, the composition of the vapor and liquid leaving the separator can be obtained