Aerobic oxidations in the production of fine chemicals & pharmaceuticals
Very often, H2O is formed as the only byproduct of aerobic oxidation reactions. This is especially advantageous for the production of active pharmaceutical ingredients (APIs), where the impurities allowed in the products are tightly regulated. The ability to deploy aerobic oxidations would alleviate substantial operational and environmental costs associated with the work-up of post-reaction mixtures, which can consume more material (particularly solvents24) and energy than the reactions themselves.25 However, in a review of large-scale oxidation processes in the pharmaceutical industry, it was concluded that: “Oxygen gas has the advantage of being the least expensive and most readily available oxidant, but the safety issues surrounding its use make it one of the least frequently used reagents”.
The key reason for the lack of aerobic oxidation reactions in the fine chemicals and pharmaceutical sectors can mainly be attributed to differences in scale and mode of operation. Economy of scale allows bulk chemical processes to be performed in dedicated facilities in a highly integrated manner, with attendant operational efficiencies and synergies. In comparison, pharmaceutical and fine chemicals are typically produced in multiple steps in limited quantities, using multipurpose batch reactors in the liquid phase. Process flexibility is therefore often achieved at the expense of efficiency. Aerobic oxidations fall into a unique category of reactions that simply cannot be performed in batch, due to the considerable headspace within the reactors.
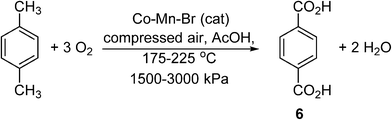
To illustrate these differences, we shall consider the current industrial process used to produce terephthalic acid (TA), (6) (Scheme 4). Contrary to many other petrochemical oxidation reactions, TA is produced industrially using an organic solvent (acetic acid), and thus may serve as a useful process to gain transferrable insight for the small scale processing of pharmaceuticals. Driven by global demand, the production of purified terephthalic acid (PTA) is estimated to reach 66 MMt per annum by 2025. The commercial process was developed by Amoco, involving the catalytic oxidation of p-xylene, performed using O2 as a terminal oxidant in acetic acid. Catalyzed homogeneously by Co and Mn salts in the presence of a radical initiator (Br−), the reaction is highly exothermic, liberating 2 × 108 J per kg of p-xylene consumed.
Scheme 4 Amoco process of catalytic aerobic oxidation of p-xylene to terephthalic acid (TA).
The Amoco process demonstrates that, in principle, from a (reaction) engineering point of view, there appear to be no insurmountable obstacles or operational restrictions to the performance of safe and effective partial oxidation reactions in organic solvents using oxygen. However, if one examines the process with the view to adopting this for the transformation of APIs, a number of issues emerge that need to be addressed, which are summarised below:
1. Reaction conditions: relatively harsh conditions are employed (175–225 °C and 15–30 bar), beyond the flash point of acetic acid (see Table 1 below), and are incompatible with the synthesis of highly functionalised (thermally sensitive) molecules in batch systems, intimating the need for a flow system.
Table 1 Autoignition temperatures (AITs) and flashpoints of commonly-used organic solvents at atmospheric pressurea
|
Solvent |
AIT/°C |
Flash point/°C |
|
Acetic acid |
427 |
40 |
|
Dichloromethane |
556 |
Noneb |
|
Diethyl ether |
160 |
−45 |
|
Methyl tert-butyl ether |
443 |
−28 |
|
N,N-Dimethylformamide |
445 |
58 |
|
N,N-Dimethylacetamide |
490 |
63 |
|
Ethyl acetate |
410 |
−3.3 |
|
Butyl acetate |
421 |
22 |
|
Tetrahydrofuran |
321 |
−17.2 |
|
2-Methyltetrahydrofuran |
270 |
−23 |
|
Ethanol |
365 |
16.6 |
|
Isopropanol |
399 |
12 |
|
Toluene |
530 |
6 |
|
p-Xylene |
530 |
27 |
|
n-Hexane |
225 |
−26 |
|
n-Heptane |
215 |
−4.0 |
|
a Obtained from MSDS. b Dichloromethane has no flash point in a conventional (closed) tester, but forms flammable vapour–air mixtures at >100 °C. |
||
2. Selectivity: equally, high temperatures and radical reactions are also unlikely to offer the level of selectivity required for the oxidation of complex molecules. In the case of xylene, radical chemistry dictates the expected complete oxidation of the methyl groups. Under these conditions, the acetic acid solvent is also oxidised (‘solvent burning’), leading to the competitive formation of carbon oxides (CO, CO2, formaldehyde).29 This causes a significant solvent loss from the process (0.05 tonne of acetic acid consumed per tonne of TA produced).
3. Stoichiometry: high pressures are required to solubilise oxygen in the solvent. For kinetic reasons the temperature also needs to be increased, reducing in turn the solubility of oxygen in the solvent. This can lead to sub-stoichiometric availability of oxygen and thus undesirable degrees of oxidation.
4. Safety: mixtures of pure oxygen and organic solvents can potentially generate highly flammable mixtures.30 In the Amoco process, the dedicated reactor is designed such that the O2 content of the off-gas is kept near to 0%. The resultant O2 deficiency can lead to a significant compromise in selectivity. Dilution of oxygen with inert gases to counteract this leads, in turn, to sub-stoichiometry and large reactor sizes.
5. Workup: TA precipitates from the reaction mixture, forming a three-phase mixture of solid product, liquid reactants/solvents and a vapour phase containing unreacted oxygen. Product separation and purification are highly optimised through a series of recrystallizations at different pressures/temperatures, which are not always possible using multipurpose reactors.
6. Capital investment: the highly corrosive bromine-acetic acid environment requires special materials to be used in the construction of reaction vessels, e.g.titanium or special alloys, which are not easily available as standard process equipment in the pharmaceutical industry.
The additional complexity and higher-purity requirements for pharmaceutical products pose two main challenges for the development of aerobic oxidation processes: (i) identification of catalysts that are able to catalyse different oxidative reactions chemoselectively under mild conditions; and (ii) design of reactors and process integration that are compatible with the use of organic solvents, and that can deliver O2 efficiently and safely without compromising catalyst activity and selectivity.31
In this Review, we present the hazards and safety issues of aerobic oxidation reactions in the liquid phase using organic solvents, followed by a brief survey of the types of reactors that can be used. This is followed by examples of aerobic oxidation reactions that are currently achieved on a large scale in industrial laboratories, particularly for the production of speciality chemicals and APIs. In each case, safety aspects of the reactions are highlighted. Finally, the challenges and future directions and opportunities are identified.