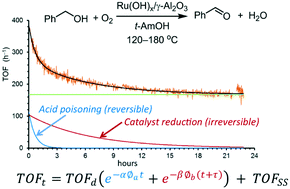
Catalysis in flow: O2 effect on the catalytic activity of Ru(OH)x/γ-Al2O3 during the aerobic oxidation of an alcohol
Abstract
Changes in the turnover frequency (TOF) of Ru(OH)x/γ-Al2O3 during aerobic oxidation of benzyl alcohol in a plug flow differential reactor were monitored online using an inline FTIR instrument over extended periods of time (up to 72 h). A new equation for catalyst deactivation is derived, to account for the different lengths of time the catalyst is exposed to the reactants (benzyl alcohol and O2). Catalyst activity and stability are dependent on the amount of O2 in the system; catalyst deactivation can be attributed to two simultaneous processes: a fast and reversible inhibition by the benzoic acid side product and a slower and irreversible loss of catalytic sites due to reduction of Ru. At steady state, the reaction rate is zero-order in benzyl alcohol and partial positive order in O2. Finally, the suitability of a packed-bed (integral) reactor for the reaction is discussed.
Decay kinetics of sensitive bioinorganic species in a Super Focus mixer at ambient conditions
Abstract
We performed studies on the oxygenation of a copper(I) complex targeting the intrinsic kinetics of this reaction in a continuous flow setup. It is an example reaction for the metal-based activation of dioxygen. For these experiments we used a SuperFocus mixer, a customised micro mixer with fast mixing behaviour. By means of this setup, we were able for the first time to detect the formation and decay of a thermally very sensitive bis(μ-oxo)dicopper species at ambient temperature. Comparing these data to results from a stopped-flow setup we could confirm the performance of the SuperFocus mixer setup. The rate constant for the decay of the bis(μ-oxo)dicopper species was determined to be 0.90 s−1using the SuperFocus mixer and to be 1.57 s−1 by stopped-flow at slightly different temperatures.

Adenine as an organ catalyst for the ring-opening polymerization of lactide: scope, mechanism and access to adenine-functionalized polylactide
Abstract
Nucleobase-functionalized polymers are widely used in the fields of supramolecular chemistry and self-assembly, and their development for biomedical applications is also an area of interest. They are usually synthesized by tedious multistep procedures. In this study, we assess adenine as an organoinitiator/organocatalyst for the ring-opening polymerization of lactide. L-Lactide can be quantitatively polymerized in the presence of adenine. Reaction conditions involving short reaction times and relatively low temperatures enable the access to adenine end-capped polylactide in a simple one-step procedure, in bulk, without additional catalyst. DFT calculations show that the polymerization occurs via hydrogen bond catalysis. The mechanism involves (i) a hydrogen bond between the NH9 of adenine and the carbonyl moiety of lactide, leading to an electron deficient carbon atom, and (ii) a second hydrogen bond between the N3 of adenine and the NH2 of a second adenine molecule, followed by a nucleophilic attack of the latter activated amine on the former electron deficient carbon on the monomer. For longer reaction times and higher temperatures, macrocyclic species are formed, and a mechanism involving the imidazole ring of adenine is proposed based on literature studies. Depending on the reaction conditions, adenine can thus be considered as an organoinitiator or an organocatalyst for the ring-opening polymerization of lactide.