Development of a reactor with carbon catalysts for modular-scale, low-cost electrochemical generation of H2O2
Abstract
The development of small-scale, decentralized reactors for H2O2 production that can couple to renewable energy sources would be of great benefit, particularly for water purification in the developing world. Herein, we describe our efforts to develop electrochemical reactors for H2O2 generation with high Faradaic efficiencies of >90%, requiring cell voltages of only ∼1.6 V. The reactor employs a carbon-based catalyst that demonstrates excellent performance for H2O2 production under alkaline conditions, as demonstrated by fundamental studies involving rotating-ring disk electrode methods. The low-cost, membrane-free reactor design represents a step towards a continuous, modular-scale, de-centralized production of H2O2.
Tuning reaction products by constrained optimisation
Abstract
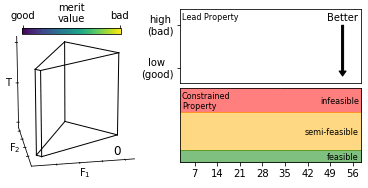
We describe an effective means of defining optimisation criteria for self-optimising reactors, applicable to situations where a compromise is sought between several competing objectives. The problem is framed as a constrained optimisation, in which a lead property is optimised subject to constraints on the values that other properties may assume. Compared to conventional methods (using weighted-sum- and weighted-product-based merit functions), the approach described here is more intuitive, easier to implement, and yields an optimised solution that more faithfully reflects user preferences. The method is applied here to the synthesis of o-xylenyl adducts of Buckminsterfullerene, using a cascadic reaction of the form X0 → X1 → X2 → … XN. Specifically, we selectively target the formation of the (technologically useful) first- and second-order adducts X1 and X2, while at the same time suppressing the formation of unwanted higher-order products. More generally, the approach is applicable to any chemical optimisation involving a trade-off between competing criteria. To assist with implementation we provide a self-contained software package for carrying out constrained optimisation, together with detailed tutorial-style instructions.
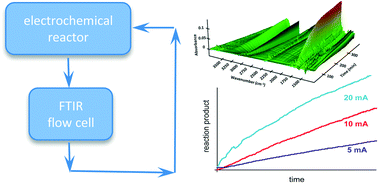
In situ FTIR spectroscopic monitoring of electrochemically controlled organic reactions in a recycle reactor
Abstract
An electrochemical cell coupled with a recycle loop through a transmission FTIR cell is employed in studies of two free radical organic reactions, the oxidation of allylic alcohols and the trifluoromethylation of heteroarenes. Rapid mixing through the recycle loop allows continuous monitoring of reaction progress. Electrochemical generation of free radicals allows their controlled mediation into the reaction mixture for more efficient reaction. Kinetic profiles provide mechanistic insight into reactions under electrochemical control.