Poly(oxymethylene) dimethyl ether synthesis – a combined chemical equilibrium investigation towards an increasingly efficient and potentially sustainable synthetic route
Abstract
Polyoxymethylene dimethyl ethers (denoted hereon as OME) are potential sustainable fuels (e.g. as a diesel substitute). In this paper, the fundamental analysis of a potentially, sustainable synthetic OME system is presented (i.e. based on CH3OH synthesised from H2and recycled CO2). In this context, a multicomponent thermodynamic vapour–liquid equilibrium model, based on CH3OH as the educt and source of H2CO for OME synthesis, is described. A thermodynamic equilibrium mathematical model for this complex (i.e. a 29 reaction network) CH3OH–H2CO equilibrium system is presented, capable of solving the sequential chemical and phase equilibrium, importantly considering all components in the reaction system including poly(oxymethylene) hemiformals and poly(oxymethylene) glycols. A theoretical efficiency evaluation indicates that the proposed anhydrous route is potentially more attractive than the conventional synthesis (i.e. based on dimethoxymethane and trioxane). To substantiate these theoretical investigations, a complimentary experimental batch OME synthesis is also presented, providing validation for the presented thermodynamic model. An initial kinetic analysis of the OME synthesis over different commercial catalysts is also highlighted. Our presented findings reliably describe the synthesis equilibrium with respect to our experimentally obtained results. The presented work supports further an operating OME synthesis framework based on CH3OH and H2CO and highlights the requirement for innovative process design regarding feed preparation, reactor technology, and product separation/fractions recycling.
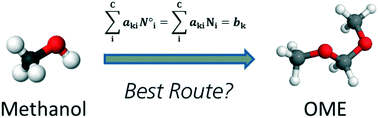
Rapid, selective and stable HaloTag-LbADH immobilization directly from crude cell extract for the continuous biocatalytic production of chiral alcohols and epoxides
Abstract
A strategy for biocatalyst immobilization in flow directly from the crude cell extract is described. The efficiency and the stability of the immobilized enzyme were demonstrated during the asymmetric reduction of a range of ketones. The cascade two-step chemo-enzymatic preparation of chiral epoxides was possible through the initial ketone bio reduction to an intermediate halohydrin followed by its intramolecular cyclization.
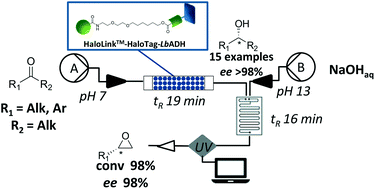
The application of reaction engineering to biocatalysis
Abstract
Biocatalysts is a growing area of synthetic and process chemistry with the ability to deliver not only improved processes for the synthesis of existing compounds, but also new routes to new compounds. In order to assess the many options and strategies available to an engineer developing a new biocatalytic process, it is essential to carry out a systematic evaluation to progress rapidly and ensure decisions are made on firm foundations. In this way, directed development can be carried out and the chances of implementation of a commercially successful process can be much improved. In this review we outline the benefits of reaction engineering in this development process, with particular emphasis of reaction kinetics. Future research needs to focus on rapid methods to collect such data at sufficient accuracy that it can be used for the effective design of new biocatalytic processes.