Development of a reactor with carbon catalysts for modular-scale, low-cost electrochemical generation of H2O2
Abstract
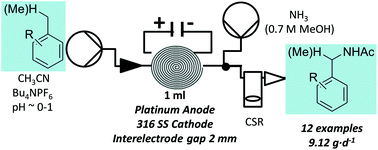
The development of small-scale, decentralized reactors for H2O2 production that can couple to renewable energy sources would be of great benefit, particularly for water purification in the developing world. Herein, we describe our efforts to develop electrochemical reactors for H2O2 generation with high Faradaic efficiencies of >90%, requiring cell voltages of only ∼1.6 V. The reactor employs a carbon-based catalyst that demonstrates excellent performance for H2O2 production under alkaline conditions, as demonstrated by fundamental studies involving rotating-ring disk electrode methods. The low-cost, membrane-free reactor design represents a step towards a continuous, modular-scale, de-centralized production of H2O2.
Aerobic oxidations in flow: opportunities for the fine chemicals and pharmaceuticals industries
Abstract
Molecular oxygen is without doubt the greenest oxidant for redox reactions, yet aerobic oxidation is one of the most challenging to perform with good chemoselectivity, particularly on an industrial scale. This collaborative review (between teams of chemists and chemical engineers) describes the current scientific and operational hurdles that prevent the utilisation of aerobic oxidation reactions for the production of speciality chemicals and active pharmaceutical ingredients (APIs). The safety aspects of these reactions are discussed, followed by an overview of (continuous flow) reactors suitable for aerobic oxidation reactions that can be applied on scale. Some examples of how these reactions are currently performed in the industrial laboratory (in batch and in flow) are presented, with particular focus on the scale-up strategy. Last but not least, further challenges and future perspectives are presented in the concluding remarks.

The application of reaction engineering to biocatalysis
Abstract
Biocatalysis is a growing area of synthetic and process chemistry with the ability to deliver not only improved processes for the synthesis of existing compounds, but also new routes to new compounds. In order to assess the many options and strategies available to an engineer developing a new biocatalytic process, it is essential to carry out a systematic evaluation to progress rapidly and ensure decisions are made on firm foundations. In this way, directed development can be carried out and the chances of implementation of a commercially successful process can be much improved. In this review we outline the benefits of reaction engineering in this development process, with particular emphasis of reaction kinetics. Future research needs to focus on rapid methods to collect such data at sufficient accuracy that it can be used for the effective design of new biocatalytic processes.
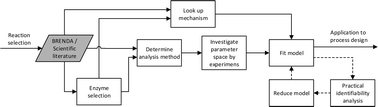
Continuous direct anodic flow oxidation of aromatic hydrocarbons to benzyl amides
Abstract
The continuous production of benzyl amides by anodic oxidation in flow was developed. The stability and productivity of the equipment was examined over time and monitored by means of in-line UV analysis. The applicability of the method to twelve substrates was demonstrated.