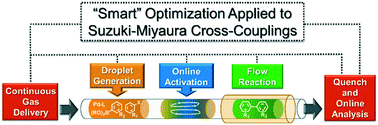
Telescoped continuous flow generation of a library of highly substituted 3-thio-1,2,4-triazoles
Abstract
We report herein the successful application of continuous flow micro reactors to prepare important building blocks based on the 3-thio-1,2,4-triazole core. A telescoped continuous flow process was developed based on the condensation of hydrazides and isothiocyanates to deliver an in situ stream of a thiosemicarbazone, which subsequently was cyclized under basic conditions. The obtained 1,2,4-triazole-3-thiol was further alkylated with benzyl/alkyl halides. In addition, we evaluated the scope of heterocycle formation and alkylation using different hydrazides, isothiocyanates, and aryl/alkyl chlorides, bromides and iodides. We were able to synthesize a small library of 18 compounds in 48 minutes of residence time for each synthesis, and in moderate to excellent yields, in a telescoped fashion. The fully integrated synthesis flow platform enables the fast generation of compound libraries, reducing the time consumed in preliminary stages of a drug discovery process.
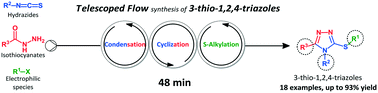
A miniature CSTR cascade for continuous flow of reactions containing solids
Abstract
Continuous handling of solids creates challenges for realizing continuous production of pharmaceuticals and fine chemicals. We present a new miniature continuous stirred-tank reactor (CSTR) cascade to handle solid-forming reactions in flow. Single-phase residence time distribution (RTD) measurements of the CSTR cascade reveal nearly ideal CSTR mixing behavior of the individual units. Consistency of experimental and predicted conversions of a Diels–Alder reaction further confirms the CSTR performance. Two solid-forming reactions, (i) glyoxal reacting with cyclohexylamine to form N,N′-dicyclohexylethylenediimine, (ii) sulfonylation of 2-octanol with methanesulfonyl chloride, demonstrate the ability of the reactor cascade to transport solid particles continuously for hours without significant signs of clogging.
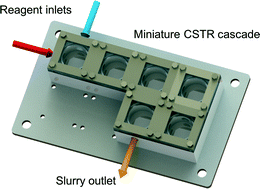
Halogenation of organic compounds using continuous flow and microreactor technology
Abstract
The halogenation of organic substrates is one the most important transformations in organic synthesis. The most straightforward, inexpensive and atom economic halogenations involve the use of elemental halogens (X2) or hydrogen halides (HX). However, X2 and HX reagents are highly reactive, toxic and corrosive materials. Halogenations using these reagents are usually very fast and exothermic reactions, in which selectivity issues occur. Using continuous flow chemistry halogenations involving X2 and HX can be performed in a safe and controllable manner. Reagents can be accurately dosed even for gas/liquid reactions, and exotherms are easily controlled. Hazardous chemicals can be readily quenched in line avoiding any undesired exposures and significantly enhancing the process safety.
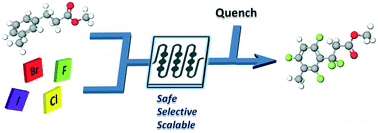
Suzuki–Miyaura cross-coupling optimization enabled by automated feedback
Abstract
An automated, droplet-flow microfluidic system explores and optimizes Pd-catalyzed Suzuki–Miyaura cross-coupling reactions. A smart optimal DoE-based algorithm is implemented to increase the turnover number and yield of the catalytic system considering both discrete variables—palladacycle and ligand—and continuous variables—temperature, time, and loading—simultaneously. The use of feedback allows for experiments to be run with catalysts and under conditions more likely to produce an optimum; consequently complex reaction optimizations are completed within 96 experiments. Response surfaces predicting reaction performance near the optima are generated and validated. From the screening results, shared attributes of successful precatalysts are identified, leading to improved understanding of the influence of ligand selection upon transmetalation and oxidative addition in the reaction mechanism. Dialkylbiarylphosphine, trialkylphosphine, and bidentate ligands are assessed.