Design protocol of microjet mixers for achieving desirable mixing times with arbitrary flow rate ratios
Abstract
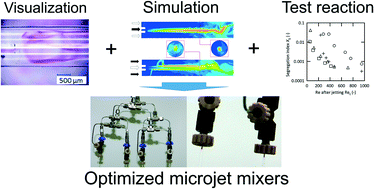
We extensively examined and discussed the performance of microjet mixers that consisted of two concentrically arranged tubes. The orientation of the feed streams with respect to the inner and outer inlets was the crucial factor for promoting the desired reaction. The Reynolds number after jetting and the velocity ratio of the inner and outer fluids were the key variables for promoting vigorous jetting and efficient mixing. By choosing the appropriate operating conditions, the microjet mixers exhibited excellent performance. The design protocol for microjet mixers was established based on mixing time measurements. We successfully maximized the throughput in one channel and numbered-up the microjet mixers. The microjet mixers also proved to be useful for treating viscous fluids with the appropriate choice of the feed orientation. The complete mixing of water with a 200 times more viscous fluid was achieved in 20 ms.
New methods for the preparation of nanoscale nickel phosphide catalysts for heteroatom removal reactions
Abstract
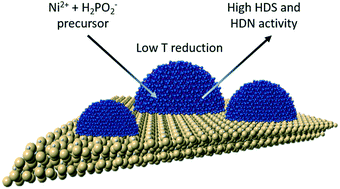
The removal of heteroatom impurities (S, N, O) from fossil fuel and biomass feedstocks is a critical step in the production of clean-burning transportation fuels. Nickel phosphide (Ni2P) has emerged as a leading candidate material on which to base a new class of catalysts to compete with the long established Ni–Mo and Co–Mo sulfides for hydrotreating of crude oil feedstocks. A recent assessment by researchers at ExxonMobil suggested that a Ni2P catalyst having a 3 nm particle size could compete with a state-of-the-art Co–Mo/Al2O3catalyst for the hydrodesulfurization (HDS) of a straight-run gas oil feed. This article reviews recent advances in the low-temperature preparation of highly-active, nanoscale Ni2P catalysts.
Synthesis and molecular weight control of poly(3-hexylthiophene) using electrochemical polymerization in a flow microreactor
Abstract
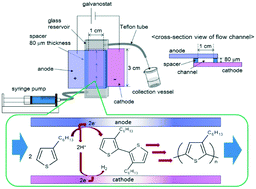
A new approach for the synthesis and molecular weight control of poly(3-hexylthiophene) (P3HT) using electrochemical polymerization in a flow microreactor is described. This synthetic system enabled electrochemical synthesis of soluble P3HT without its deposition using a flow microreactor. Careful selection of the reaction conditions enabled the control of molecular weight and distribution of the synthesized P3HT.
Design and testing of an operando-Raman annular reactor for kinetic studies in heterogeneous catalysis
Abstract
In this work, we present a novel experimental tool that integrates in situ Raman spectroscopy and an annular reactor for the operando-Raman kinetic analysis of heterogeneous catalytic reactions. The proposed configuration can monitor via Raman spectroscopy the catalytic surface under kinetically limited reaction conditions, with reliable product analysis, thus retaining the main features of both Raman spectroscopy and kinetic investigation in an annular reactor. We report a thorough description of the key constraints in developing online Raman spectroscopic tools for kinetic investigations. These constraints are considered in the design, assembly and testing of the experimental method by minimizing the mutual invasiveness of the Raman spectroscopy and of the annular reactor configurations. Show-cases of dry reforming and partial oxidation of CH4 on Rh catalysts are used to establish proof of concept of the method, demonstrating the acquisition of time-resolved Raman spectroscopic data under kinetically relevant conditions. Experiments both on clean and coked Rh surfaces reveal that well-structured graphitic deposits are likely to form during DR. During CPO, instead, the presence of O2 and H2O limits the formation of organized graphitic-like carbonaceous species. On a more general basis, this reactor allows a detailed structural characterization of a catalyst material during the reaction and at conditions of temperature, pressure and composition relevant to catalysis. Therefore, it is an important breakthrough for the simultaneous collection of spectroscopic and kinetically relevant data for the investigation of the structure–activity relationship in heterogeneous catalysis.