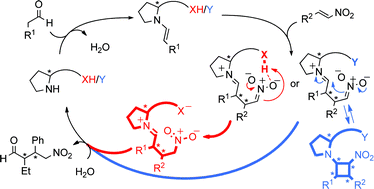
Iron-catalysed kinetic resolution of N-sulfonyl ox aziridines
Abstract
We have developed a highly selective kinetic resolution of N-sulfonyl oxaziridines. This reaction utilizes an inexpensive and easily synthesized iron bis(oxazoline) catalyst to promote the efficient rearrangement of oxaziridines to the corresponding N-sulfonyl imides; no sacrificial reagents are required to effect this resolution. This process is readily translated to gram scale, which provides a practical method for the preparation of structurally diverse, enantiopure N-sulfonyl oxaziridines for use as reagents in organic synthesis.
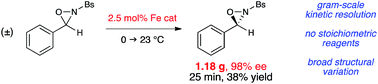
Effects of internal and external carboxylic acids on the reaction pathway of organocatalytic 1,4-addition reactions between aldehydes and nitroolefins
Abstract
Kinetic and NMR spectroscopic studies revealed that the reaction pathway of conjugate addition reactions between aldehydes and nitroolefins depends on the presence or absence of a suitably positioned carboxylic acid group within the catalyst. The intermediate nitronate is trapped intramolecularly either by protonation (in the presence of a well positioned intramolecular carboxylic acid) or by C–C bond formation to a cyclobutane intermediate (in the absence of an intramolecular proton donor). These differences in the reaction pathway are reflected in different rate limiting steps of the reaction. The studies demonstrated that the preferred reaction pathway and thereby the rate limiting steps of the reaction can be influenced by additives of different acidity or the position of an intramolecular carboxylic acid group within the catalyst.