Hydrogenation of 2-methyl-3-butyn-2-ol over a Pd/ZnO catalyst: kinetic model and selectivity study
Abstract
The three-phase hydrogenation of 2-methyl-3-butyn-2-ol has been studied over a Pd/ZnO catalyst. A Langmuir–Hinshelwood mechanism was applied assuming noncompetitive adsorption between hydrogen and organic molecules on the catalyst active sites. All experimental runs used for the modeling have been obtained in the intrinsic kinetic regime in order to exclude any mass transfer limitation. An optimization procedure allowed the estimation of the kinetic and adsorption parameters governing the process. The results revealed that the proposed model accurately describes the behavior of the system in the typical operating ranges of industrial reactors. The performance of the catalyst in terms of selectivity to 2-methyl-3-buten-2-ol and initial activity is found to be higher compared with that of a commercial Lindlar catalyst under the same operating conditions. The mathematical model, successfully validated, is able to accurately predict the selectivity of the process.
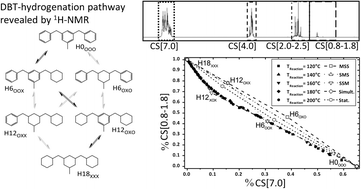
Hydrogenation of the liquid organic hydrogen carrier compound dibenzyltoluene – reaction pathway determination by 1H NMR spectroscopy
Abstract
The catalytic hydrogenation of the LOHC compound dibenzyltoluene (H0-DBT) was investigated by 1H NMR spectroscopy in order to elucidate the reaction pathway of its charging process with hydrogen in the context of future hydrogen storage applications. Five different reaction pathways during H0-DBT hydrogenation were considered including middle-ring preference (middle-side-side, MSS), side-middle-side order of hydrogenation (SMS), side-ring preference (SSM), simultaneous hydrogenation of all three rings without intermediate formation and statistical hydrogenation without any ring preference. Detailed analysis of the 1H NMR spectra of the H0-DBT hydrogenation over time revealed that the reaction proceeds with a very high preference for the SSM order at temperatures between 120 °C and 200 °C and 50 bar in the presence of a Ru/Al2O3-catalyst. HPLC analysis supported this interpretation by confirming an accumulation of H12-DBT species prior to full hydrogenation to H18-DBT with middle ring hydrogenation as the final step.