A convenient numbering-up strategy for the scale-up of gas–liquid photo redox catalysis in flow
Abstract
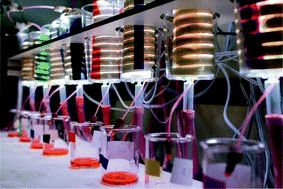
Visible-light photocatalysis is a mild activation method for small molecules and enables a wide variety of transformations relevant for organic synthetic chemistry. However, one of the limitations of photocatalysis and photochemistry in general is the limited scalability due to the absorption of light (Lambert–Beer law). Here, we report the development of a convenient numbering-up strategy for the scale-up of gas–liquid photocatalytic reactions in which the gas is consumed. Only commercially available constituents were used and the system can be rapidly assembled by any practitioner of flow chemistry. The modular design allows us to systematically scale the photochemistry within 2n parallel reactors (herein, n = 0, 1, 2, 3). The flow distribution in the absence of reactions was excellent, showing a standard deviation less than 5%. Next, we used the numbered-up photomicroreactor assembly to enable the scale-up of the photocatalytic aerobic oxidation of thiols to disulfides. The flow distribution was again very good with a standard deviation lower than 10%. The yield of the target disulfide in the numbered-up assemblies was comparable to the results obtained in a single device demonstrating the feasibility of our approach.
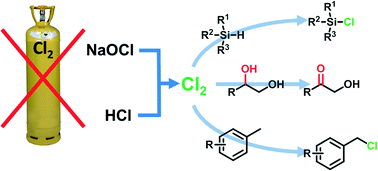
A laboratory-scale continuous flow chlorine generator for organic synthesis
Abstract
A simple continuous flow setup for the generation and use of elemental chlorine for organic synthesis has been developed. The chlorine generator is based on the reaction of HCl with NaOCl, generating NaCl and H2O as the only side products. As a proof-of-concept, the reactor has been applied for a variety of chlorinations and oxidations of organic compounds.
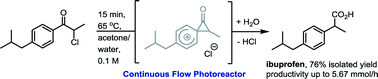
Continuous photochemistry: the flow synthesis of ibuprofen via a photo-Favorskii rearrangement
Abstract
A new enabling technology for performing photochemical reactions in a continuous fashion is presented. This photo-reactor is compatible with existing flow systems and can be furthermore linked to a photo-spectrometer in order to allow for real time analysis of photochemical reactions. In this communication we wish to report the profiling of this system and its application to the continuous synthesis of ibuprofen based on a photo-Favorskii rearrangement reaction of a readily available α-chloropropiophenone precursor.
A multistep continuous flow synthesis machine for the preparation of pyrazoles via a metal-free amine-redox process
Abstract
A versatile multistep continuous flow setup is reported for the four-step conversion of anilines into pyrazole products. The synthesis machine incorporates the use of amine-redox chemistry through diazotization and a metal-free vitamin C mediated reduction. The machine can be used for the synthesis of an array of analogues or the scale up of an individual target.
