Elementary Reactions
Recall from the discussion of reaction networks in Chapter 1 that an elementary reaction must be written as it proceeds at the molecular level and represents an irreducible molecular event. An elementary reaction normally involves the breaking or making of a single chemical bond, although more rarely, two bonds are broken and two bonds are formed in what is denoted a four-center reaction. For example, the reaction:
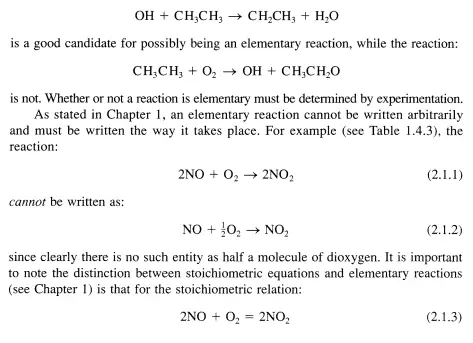
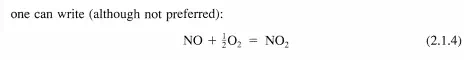
The remainder of this chapter describes methods to determine the rate and temperature dependence of the rate of elementary reactions. This information is used to describe how reaction rates in general are appraised.