General Properties of the Rate Function for a Single Reaction
The rate of reaction is generally a function of temperature and composition, and the development of mathematical models to describe the form of the reaction rate is a central problem of applied chemical kinetics. Once the reaction rate is known, infonnation can often be derived for rates of individual steps, and reactors can be designed for carrying out the reaction at optimum conditions.
Below are listed general rules on the fonn of the reaction rate function (M. Boudart, Kinetics of Chemical Processes, Butterworth-Heinemann, 1991, pp. 13-16). The rules are of an approximate nature but are sufficiently general that exceptions to them usually reveal something of interest. It must be stressed that the utility of these rules is their applicability to many single reactions.
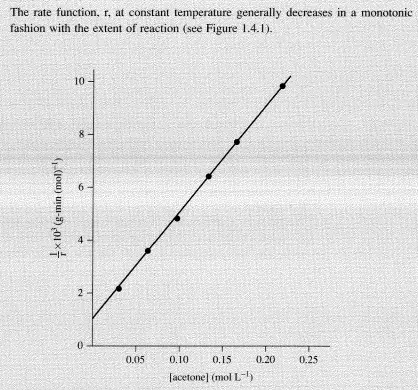
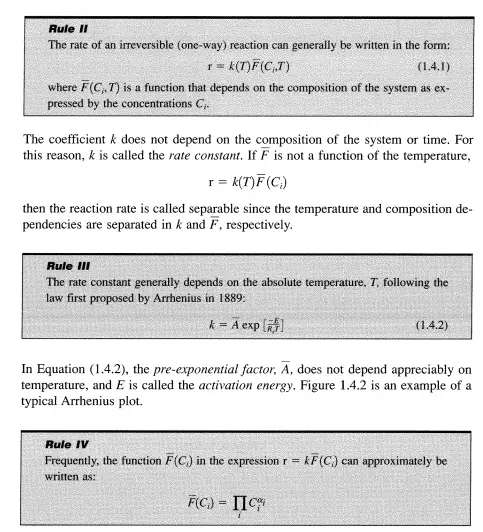
The product n is taken over all components of the system. The exponents Ui are small integers or fractions that are positive, negative, or zero and are temperature independent at least over a restricted interval.