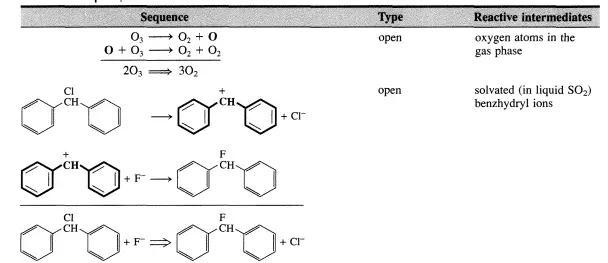
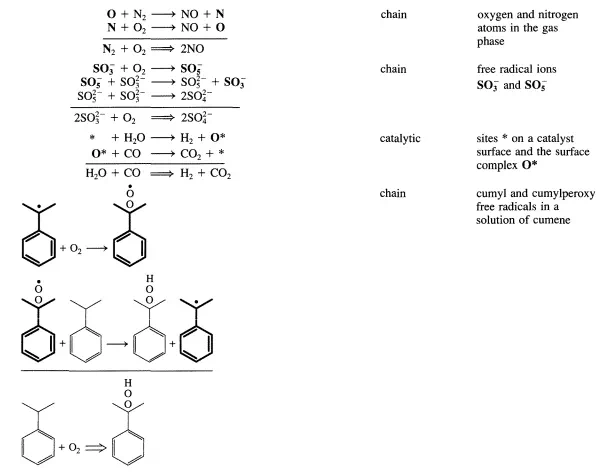
Single Reactions
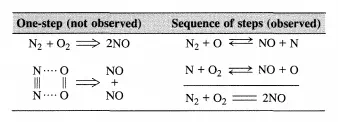
One-step reactions between stable molecules are rare since a stable molecule is by definition a quite unreactive entity. Rather, complicated rearrangements of chemical bonds are usually required to go from reactants to products. This implies that most reactions do not proceed in a single elementary step as illustrated below for NO formation from Nz and Oz:
Normally, a sequence of elementary steps is necessary to proceed from reactants to products through the formation and destruction of reactive intermediates (see Section 1.1). Reactive intermediates may be of numerous different chemical types (e.g., free radicals, free ions, solvated ions, complexes at solid surfaces, complexes in a homogeneous phase, complexes in enzymes). Although many reactive intermediates may be involved in a given reaction (see Scheme 1.1.1), the advancement of the reaction can still be described by a single parameter-the extent of reaction (see Section 1.2). If this is the case, the reaction is said to be single. Why an apparently complex reaction remains stoichiometrically simple or single, and how the kinetic treatment of such reactions can be enumerated are the two questions addressed in this chapter.
There are two types of sequences leading from reactants to products through reactive intermediates. The first type ofsequence is one where a reactive intermediate is not reproduced in any other step of the sequence. This type of sequence is denoted as an open sequence. The second type of sequence is one in which a reactive intermediate is reproduced so that a cyclic reaction pattern repeats itself and a large number of product molecules can be made from only one reactive intermediate. This type of sequence is closed and is denoted a catalytic or chain reaction cycle. This type of sequence is the best definition of catalysis.
A few simple examples of sequences are listed in Table 4.1.1. The reactive intermediates are printed in boldface and the stoichiometrically simple or single reaction is in each case obtained by summation of the elementary steps of the sequence. While all reactions that are closed sequences may be said to be catalytic, there is a distinct difference between those where the reactive intermediates are provided by a separate entity called the catalyst that has a very long lifetime and those where the reactive intermediates are generated within the system and may survive only during a limited number of cycles.
The first category encompasses truly catalytic reactions (catalytic reaction cycle) in the narrow sense of the word, while the second involves chain reactions (chain reaction cycle). Both types exhibit slightly different kinetic features. However, the two types are so closely related that it is conceptually straightforward to consider them together. In particular, both categories can be analyzed by means of the steady-state approximation that will be presented in the next section. Chain and catalytic reaction cycles provide energetically favorable pathways for reactant molecules to proceed to product molecules.
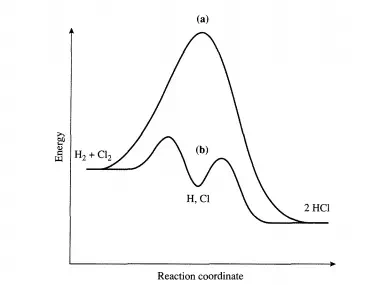
This point is illustrated below for both types of cycles. Consider the reaction between dihydrogen and dichlorine to produce HCl that can be brought about in the gas phase by irradiating the reactants with light. It is known that over 106 molecules of HCl can be formed per absorbed photon. The reaction proceeds as follows:
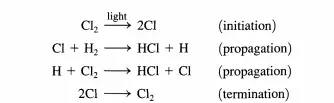
Once chlorine atoms are produced (initiation), the propagation steps provide a closed cycle that can be repeated numerous times (e.g., 106 ) prior to the recombination of the chlorine atoms (termination). The reason the chain reaction cycle dominates over a direct reaction between dihydrogen and dichlorine is easy to understand.
The direct reaction between Hz and Clz has an activation energy of over 200 kJ/mol, while the activation energies of the two propagation steps are both less than 30 kJ/mol (see Figure 4.1.1). Thus, the difficult step is initiation and in this case is overcome by injection of photons. Now consider what happens in a catalytic reaction cycle. For illustrative purposes, the decomposition of ozone is described. In the presence of oxygen atoms, ozone decomposes via the elementary reaction:
![]()