Measurement of Reaction Rates
What are the types of problems that need to be addressed by measuring reaction rates? The answers to this question are very diverse. For example, in the testing of catalysts, a new catalyst may be evaluated for replacement of another catalyst in an existing process or for the development of a new process. Accurate, reliable laboratory reaction rate data are necessary for the design of an industrial reactor
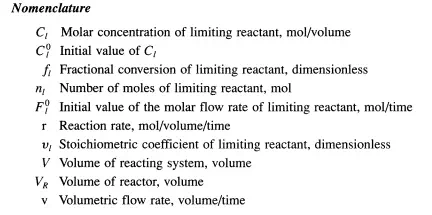
whether the process is new or old. Another example of why reaction rate data are needed is to make predictions about how large-scale systems behave (e.g., the appearance of ozone holes and the formation of smog). The key issue in all of these circumstances is the acquisition of high-quality reaction rate data. In order to do this, a laboratory-scale reactor must be used. Although deviations from ideal behavior still exist in laboratory reactors, deliberate efforts can be made to approximate ideal conditions as closely as possible. Table 3.5.1 summarizes the material balance equations for the ideal reactors described above. Examples of how these types of reactors are used to measure reaction rates are presented below.
When choosing a laboratory reactor for the measurement of reaction rate data, numerous issues must be resolved. The choice of the reactor is based on the characteristics of the reaction and for all practical matters by the availability of resources (i.e., reactors, analytical equipment, money, etc.). A good example of the issues involved in selecting a laboratory reactor and how they influence the ultimate choice is presented by Weekman [AIChE J., 20 (1974) 833]. Methods for obtaining reaction rate data from laboratory reactors that approximate the ideal reactors listed in Table 3.5.1 are now discussed.