Insulin Production using Biotechnology
The process of insulin production using Biotechnology involves the following steps:
The gene responsible for producing human insulin is located on chromosome 11. The gene is fairly small and consists of two polypeptide chains, an A chain which codes for 21 amino acids and a B chain which codes for 30 amino acids. The both chains are linked by a disulphide bond 5.

Figure 7 illustrating the chemical structure of insulin gene
The enzyme, restriction endonuclease splices the DNA at specific nucleotide bases in order to isolate the gene. This process is called restriction digestion and results in the formation of sticky or blunt ends.

Figure 8 illustrating the splicing of the DNA with the restriction endonuclease enzyme.
Another method may involve using the mRNA for the insulin gene and reverse transcriptase to produce a complementary/ copy DNA (cDNA) strand. Further explanation of this method can be found at http://www.littletree.com.au/dna.htm

Figure 9 showing translation of mRNA
A plasmid, which is a circular piece of DNA, is removed from the bacteria Escherichia coli and purified. The same restriction endonuclease enzyme is used to splice the plasmid ring .This produces complementary sticky ends to which the insulin gene can be attached.5 The plasmid is referred to as a vector.
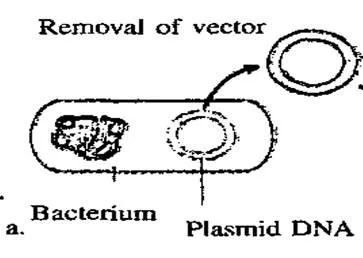
Figure 10 illustrating the removal of the plasmid from the bacterium
The human insulin gene is ligated next to the lacZ gene on the plasmid, thus forming a recombinant plasmid. The A and B chains are inserted separately into different plasmids. LacZ encodes for B-galactosidase and is widely used in recombinant DNA procedures. This is due to the fact that it is easy to locate and splice, allowing the insulin produced to be readily removed so that it does not get lost in the bacterium's DNA. Adjacent to this gene, is the amino acid methionine, which initiates insulin formation.
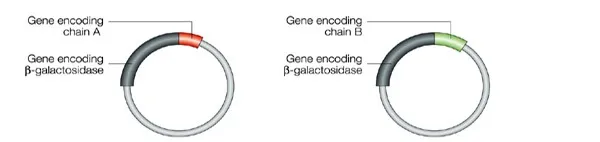
Figure 11 illustrating the recombinant plasmids
The bacterial cell is made competent either by electroporation or by using a cold liquid, such as CaCl2 (calcium chloride) to temporarily induce a more permeable cell membrane. The recombinant plasmids and the bacterial cells are then mixed together. Plasmids enter the bacteria in a process called transformation.
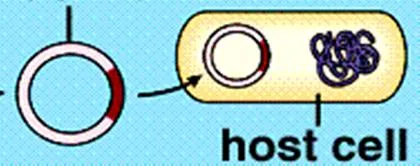
Figure 12 illustrating the plasmid being inserted back into the bacterial host cell.
The recombinant plasmid replicates autonomously within the host cell resulting in the creation of numerous identical copies.
Reproduction of bacterial host cells
At optimal temperatures within large tanks, in manufacturing industries, the bacterial cells reproduce mitotically every 20 minutes. Each daughter cell contains a recombinant plasmid and expresses the gene for human insulin 2.

Figure 13 showing replication of bacterial cell.
After cloning, the bacterial cells are removed from the tanks and lysed in order to extract the protein. A popular method involves first adding a mixture of lysozome that digests the outer layer of the cell wall, then adding a detergent mixture that separates the fatty cell wall membrane. The bacterium's DNA is then treated with cyanogen bromide, a reagent that splits protein chains at the methionine residues. This separates the insulin chains from the rest of the DNA.
The two chains are then mixed together and joined by disulfide bonds through the reduction-reoxidation reaction. An oxidizing agent is added and then the batch is placed in a centrifuge.
The mixture is then purified so that only the insulin chains remain. This can be done using several chromatography or separation techniques. The insulin batches are usually tested to ensure that none of the bacteria's E. coli proteins are mixed in with them. A marker protein that can detect E. coli DNA is used 7.