Gluconic Acid
In industrial microbiology gluconic acid has a long history. Alsberg in 1911 explained the production of gluconic acid in which Pseudomonas was used as fermenting microorganism. In 1928, its production using the fungus Penicillium luteum by surface fermentation process was employed for the first time.
Gluconic acid is presently produced commercially either by employing the fungus Aspergillus niger or the bacterium, Acetobacter suboxydans through submerged fermentation process, in which gluconic acid, sodium and calcium gluconate and glucose oxidase are produced. Apart from the above two microorganisms, other organisms are also reported to produce gluconic acid, however, they are not used for commercial production. The organisms include the fungi Endomycosis, Pullularia, Penicillium, Gonatobotrys and Scopulariosis and the bacteria Pseudomonas and Vibrio.
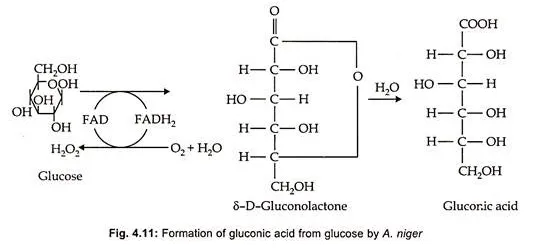
Gluconic acid is produced from glucose. This, reaction is catalysed by the enzyme glucose oxidase (Fig. 4.11).

The gluconolactone formed at the end of the first step undergoes hydrolysis either spontaneously or enzymatically to produce gluconic acid. During the first reaction, the hydrogen from FADH2 is transferred to oxygen leading to the formation of H2O2 which is immediately split into water and oxygen by the enzyme catalase, to prevent its antimicrobial activity.
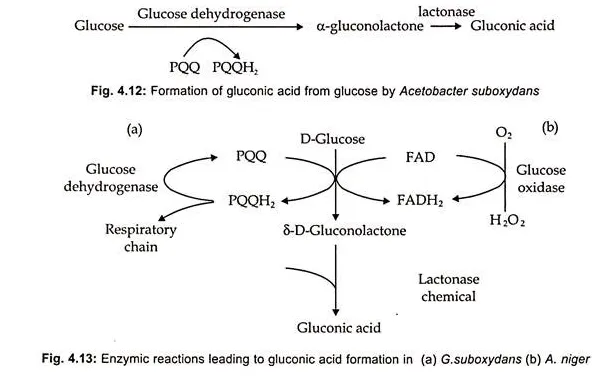
Gluconic acid is produced industrially by employing the fungus or the bacterium. In the former process Aspergillus niger and in the later process Acetobacter suboxidans (Fig. 4.12) are used. (Fig. 4.13) A. niger employs glucose oxidase involving agent like FAD and lactonase in the presence of O2 resulting in the formation of gluconic acid. On the other hand, Gluconobacter employs glucose dehydrogenase with coenzyme pyrroloquinoline quinone (PQQ) and lactonase which help in dissipating hydrogen peroxide.
(a) Fungal Fermentation:
Submerged fermentation process is employed for fungal fermentation. Aspergillus niger is used as a microorganism. Either sporulated culture or spores germinated in seed tank is used as inoculum. Each method has its own advantages. For example – use of spore inoculum directly avoids the cost of installation and operation of the seed tank, while use of germinated spores reduces operating cycles for the main fermenter.
Glucose is used as a solution or crystalline glucose or in the form of a syrup prepared from starch or crude starchy material which are treated with amylase and amyloglucosidase (table 4.6).
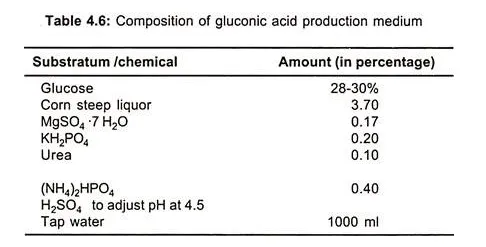
It is necessary to maintain maximum concentration of dissolved oxygen in the form of solution by vigorous agitation with the help of turbido mixture or cavitator. In the manufacture of calcium or sodium gluconate optimum temperature is maintained at 28-30°C and pH 6.5 along with vigorous agitation and aeration.
(i) Recovery and Harvest:
After fermentation, the fungal mycelium is separated by filtration, the separated mycelium is used for the recovery of glucose. On the other hand, the filtrate is used for the recovery of calcium or sodium gluconate.
(ii) Production of Calcium Gluconate:
The following steps are employed for the recovery of calcium gluconate:
1. The filtrate is heated with excess of Ca(OH)2.
2. The resulting product is decolorized with activated carbon and filtered.
3. The compound is crystallized by cooling at a temperature below 20°C and seeding with calcium gluconate crystals.
4. The mother liquor left over (about 10-15%) is used for the production of second crop of calcium gluconate through heating followed by treatment with carbon filtration and chilling.
(iii) Production of Sodium Gluconate:
Recovery of sodium gluconate is relatively simple and consists of the following steps:
1. The filtrate is concentrated to about 42-45 percent.
2. Then sodium hydroxide is added which not only crystallizes sodium gluconate but also maintains pH at 7.5.
3. The sodium gluconate thus formed is drum dried.
(iv) Production of Pure Gluconic Acid:
It is also possible to obtain gluconic acid in pure form by the following procedure, where calcium gluconate is employed:
1. Calcium is precipitated by the addition of sulfuric acid. Calcium sulfate thus formed is separated by filtration.
2. The filtrate is decolorized with activated charcoal.
3. The acid solution is concentrated to 50 percent acid strength. The product thus formed is a mixture of gluconic acid and lactones.
4. The concentrate is then treated with a temperature ranging from 0°C-30°C. The crystals that separate at this temperature consist of pure gluconic acid.
5. When the concentrate is treated with a temperature ranging from 30°-70°C crystals of lactones separate.
6. Y-lactone crystals get separated when the concentrate is treated with 70°C and above temperature.
(b) Bacterial Fermentation:
Submerged fermentation process is used. The bacterium employed is Acetobacter suboxydans or Gluconobacter suboxydans. Pure culture of the bacterium is raised by repeated sub-culturing process which is used in the fermentative production of gluconoic acid and is produced either in the form of calcium gluconate or sodium gluconate.
Glucose is used as a substratum in the fermentation. If calcium gluconate is to be produced 13-15% glucose can be added as a substrate because of low solubility of calcium gluconate (4 g liter-1 at 30°C). Higher calcium gluconate levels are formed if glucose is used above 13% levels which would spontaneously crystallize as calcium gluconate, and makes purification difficult. If sodium gluconate is to be produced, a glucose concentration of 28-30% can be used.
The fermentation is carried out at a temperature of 28-30°C, pH 4.5-6.5 with a high aeration rate of 1-1.5 vvm. The gluconic acid yield can be substantially increased (90-95%) by increasing the solubility of oxygen which is achieved by raising the pressure in the system.
1. Gluconic acid is used in the manufacture of metal, leather and food.
2. Sodium gluconate is used as a sequestering agent in many detergents.
3. Calcium gluconate is used in medicine.
4. Gluconolactone is used as baking powder and as an additive.