TCR Cell Signaling Pathway
T-cells are a subset of lymphocytes that play a large role in the immune response. The TCR (T-cell receptor) is a complex of integral membrane proteins that participate in the activation of T-cells in response to an antigen. Stimulation of TCR is triggered by MHC (major histocompatibility complex) molecules on cells with the antigen. Engagement of the TCR initiates positive and negative cascades that ultimately result in cellular proliferation, differentiation, cytokine production, and/or activation-induced cell death. These signaling cascades regulate T-cell development, homeostasis, activation, acquisition of effector’s functions and apoptosis [1, 2]. Thermo Scientific™ has a wide range of products to help with TCR research.
TNF interacts with many pathway targets, including:
ATF2 | ERK1/2 | MEKK1 |
B7-1 | Fyn | MKK3 |
B7-2 | GADS | MKK4 |
BCL10 | GRAP | MKK6 |
CARD11 | GRB2 | MKK7 |
CD3-gamma | MHC | NFAT |
CD3-delta | IKK | NF-κB |
CD3-epsilon | IL-2 | p38 |
CD3-zeta | ITAM | PAG |
CD4 | ITIM | PAK |
CD8 | JNK | Rac |
CD28 | LAT | Raf1 |
CD45 | Lck | Ras |
c-Jun | MAGUK | SHP2 |
CTLA4 | MALT1 | SIT |
Elk1 | MAPK | VHR |
ERK | MEK | ZAP70 |
TCR is composed of six different chains that form the TCR heterodimer responsible for ligand recognition. CD3 molecules are assembled together with the TCR heterodimer. CD3 possess a characteristic sequence motif for tyrosine phosphorylation, known as ITAMs (immunoreceptor tyrosine-based activation motifs). The TCR polypeptides themselves have very short cytoplasmic tails, and all proximal signaling events are mediated through the CD3 molecules. TCR-CD3 complex interaction plays an important role in mediating cell recognition events.
When TCRs are engaged by antigens the tyrosine phosphorylation of the ITAMs, present in the TCR-associated CD-zeta subunits, is triggered. Such ITAMs function by orchestrating the sequential activation of the Src-related PTKs: LcK and Fyn, which initiate TCR signaling, followed by that of ZAP70 (zeta-chain (TCR) associated protein kinase of 70 kDa), which further amplifies the response. Lck is activated by the interaction of MHC-II and CD4 or CD8. These various PTKs induce tyrosine phosphorylation of several polypeptides, including the transmembrane adaptor LAT (linker activator for T-cells). Protein tyrosine phosphorylation subsequently leads to the activation of multiple pathways, including ERK (extracellular signal regulated kinase), JNK (c-Jun N-terminal kinase), NF-κB and NFAT (nuclear factor of activated T-cells) pathways, which ultimately induce effector functions [3, 4, 5].
TCR activation is regulated by various co-stimulatory receptors. CD28 provides an essential co-stimulatory signal during T-cell activation, which augments the production of IL-2 (Interleukin-2), increases T-cell proliferation and prevents the induction of anergy and cell death. Once ligated by B7-1 or B7-2, CD28 provides the T-cell with an initial adhesion capable of approximating the T-Cell and antigen presenting cell membranes.
Besides CD28, many other transmembrane receptors also modulate specific elements of TCR signaling. CD45 is one such receptor which regulates TCR signaling by modulating the phosphorylation state of the tyrosine kinases like Lck and Fyn, and antagonizing the inhibitory impact of inhibitory proteins, thereby favoring T-cell activation.
LAT is an integral membrane adaptor protein that resides in lipid membrane rafts and binds to the adaptor GADS (growth factor receptor-bound protein-2-related adaptor protein-2), SLP76 (SH2 domain-containing leukocyte protein-76), and ITK (IL-2 inducible T-cell kinase). LAT stimulates one critical protein PLC-gamma1 (phospholipase-C-gamma1) that is responsible for the production of the second messengers DAG (diacylglycerol) and IP3 (inositol triphosphate) by cleaving PIP2 (phosphatidylinositol-4,5-bisphosphate) at the plasma membrane.
Tyrosine-phosphorylated LAT also binds multiple members of the GRB2 family of adaptor proteins, such as GRB2, GRAP (GRB2- related adaptor protein) and GADS (GRB2 -related adaptor protein-2) to facilitate the assembly of macromolecular signaling complexes that are required for efficient T-cell activation. The interaction of tyrosine-phosphorylated LAT with GRB2 provides a mechanism by which GRB2 and GRAP-associated SOS are recruited to the plasma membrane and potentially activate Ras.
TCR activation also leads to cytoskeletal rearrangements through the activation of GTP-binding proteins Rac and PAK, downstream of ZAP70 .
Activation of Ras leads to the activation of a number of serine/threonine kinases: Raf1, MEK (MAPK/ERK Kinase) and dual-specificity kinases that are responsible for the eventual upregulation of the MAPKs (mitogen activated protein kinases): ERK1/2. In contrast, ERK activation can also be downregulated by TCR signaling by phophorylated VHR (dual specificity protein phosphatase VHR), a dual specific phosphatase, phosphorylated by ZAP70.
Other MAPKs like JNK and p38 are also activated by ZAP70 and SLP76, downstream of Vav. Vav in turn phosphorylates and activates the GTP-binding protein Rac which further mediates the activation of MEKK1 (MAP/ERK Kinase Kinase-1).
p38 is activated by MEKK1 through the activation of MKK3 (mitogen-activated protein kinase kinase-3)/MKK6. JNK is activated by MEKK1 through activation of MKK4 (mitogen-activated protein kinase kinase-4)/MKK7. These MAPKs directly phosphorylate transcription factors involved in the formation of the heterodimeric transcription factor, Jun-Fos complex, as well as NFAT and IL-2 (interleukin-2) gene expression in synergy with Ca2+ signaling. ERKs, JNK and p38 directly phosphorylate the transcription factors: Elk1, c-Jun and ATF2 (activating transcription factor-2) respectively. Another transcription factor important for the generation of IL-2 is NF-κB [6, 7, 8]. NF-κB members control the expression of various genes involved in inflammatory, apoptotic and immune responses [3]. Activation of NF-κB is dependent on stimulation of the TCR and co-stimulation via CD28.
Negative regulation of TCR signaling is also significant, in order to keep a check on the hyperactivation of immune response associated with the pathway. SIT (SHP2-interacting transmembrane adaptor protein) is a recently identified transmembrane adaptor protein, which interacts with the SHP2 (SH2-containing protein tyrosine phosphatase-2) via an ITIM (immunoreceptor tyrosine-based inhibition motif), and the complex acts as a critical negative regulator of TCR-mediated signaling .
In resting human T-Cells, PAG (phosphoprotein associated with glycosphingolipid microdomains), a transmembrane adaptor molecule found in lipid rafts, is tyrosine phosphorylated and associated with CSK (c-Src tyrosine kinase), an inhibitor of Src-related protein tyrosine kinases. As a result, overexpression of PAG inhibits TCR-mediated responses. These modifications (association of PAG and CSK) are rapidly lost in response to TCR stimulation in order to maintain uninterrupted TCR signaling .
In addition, CTLA4 (cytotoxic T-lymphocyte antigen-4) also negatively regulates T-cell activation. The transmembrane protein CTLA4 also serves as a natural inhibitor. Once T-cells become activated, by whatever disease process is turning them on, the body has a natural process to turn down the T-cell pathways so that it does not get out of control.
After T-cell activation, CTLA4 is rapidly endocytosed, thus removing it rapidly from the cell surface. The significance of the apparent tight control of CTLA4 expression is due to the fact that CTLA4 has a greater affinity for its B7-1/B7-2 ligands in comparison to CD28; thus, when CTLA4 is not required, it is endocytosed, to maintain a fast T-cell activation response. When a need to control TCR signaling arises, ZAP70 activates the process of active release and translocation of CTLA4 to the membrane. At the membrane, CTLA4 interacts with the SHP2 and inhibits the phosphorylation of TCR. Another mechanism by which CTLA4 might antagonize T-cell function is through inhibition of CD28 signaling by competing for their shared ligands B7-1 and B7-2 .
Every effective immune response involves T-cell activation. T-cells are especially important in cell-mediated immunity or the defense against tumor cells and pathogenic organisms inside cells. T-cells are also involved in rejection reactions. Deregulation of T-cell function, whether by defect or by excess, results in dire consequences for the organism. These consequences could include immunodeficiency and autoimmunity, respectively.
TCR is an extremely sensitive system. Very few peptide-MHC complexes on an antigen-presenting cell are needed to trigger a T-cell response. Since T-cells play various critical roles in orchestrating the immune responses, this knowledge should lead to an understanding of how breakdowns in immune regulation lead to autoimmune diseases and of how the immune system could be better manipulated to overcome afflictions such as cancer, infection and autoimmune diseases .
Thermo Scientific™ offers antibodies, ELISAs, Luminex® multiplex assays and growth factors for key targets in the TCR signaling pathway.
Featured below is flow cytometry, western blot, and immunohistochemistry data using Thermo Scientific™ products.

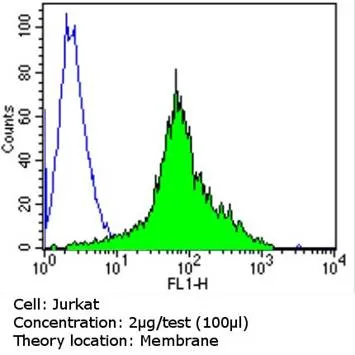
Flow cytometry analysis of TCR V alpha 12.1 in Jurkat cells (green) compared to an isotype control (blue). Cells were harvested, adjusted to a concentration of 1-5x10^6 cells/mL, fixed with 2% paraformaldehyde and washed with PBS. Cells were blocked with a 2% solution of BSA-PBS for 30 minutes at room temperature and incubated with a TCR V alpha 12.1 monoclonal antibody (Product # TCR1764) at a dilution of 2 µg/test for 60 minutes at room temperature. Cells were then incubated for 40 minutes at room temperature in the dark using a Dylight 488-conjugated goat anti-mouse IgG (H+L) secondary antibody and re-suspended in PBS for FACS analysis.
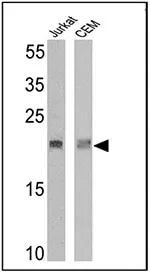
Western blot analysis of TCR V delta 2 was performed by loading 25 µg of Jurkat (lane 1) and CEM (lane 2) cell lysates onto an SDS polyacrylamide gel. Proteins were transferred to a PVDF membrane and blocked at 4°C overnight. The membrane was probed with a TCR V delta 2 monoclonal antibody (Product # TCR1732) at a dilution of 1:10 overnight at 4°C, washed in TBST, and probed with an HRP-conjugated secondary antibody for 1 hour at room temperature in the dark. Chemiluminescent detection was performed using Pierce ECL Plus Western Blotting Substrate (Product # 32132). Results show a band at ~19 kDa.
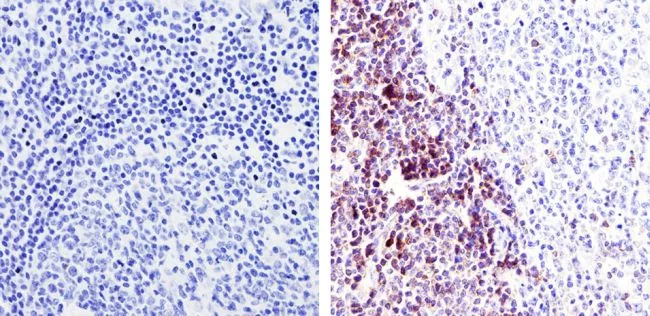
Immunohistochemistry analysis of TCR Alpha F1 showing staining in the cytoplasm of paraffin-treated human tonsil tissue (right) compared with a negative control in the absence of primary antibody (left). To expose target proteins, antigen retrieval was performed using 10mM sodium citrate (pH 6.0), microwaved for 8-15 minutes. Following antigen retrieval, tissues were blocked in 3% H2O2-methanol for 15 minutes at room temperature, washed with ddH2O and PBS, and then probed with a TCR Alpha F1 monoclonal antibody (Product # TCR1145) diluted by 3% BSA-PBS at a dilution of 1:20 overnight at 4°C in a humidified chamber. Tissues were washed extensively in PBST and detection was performed using an HRP-conjugated secondary antibody followed by colorimetric detection using a DAB kit. Tissues were counterstained with hematoxylin and dehydrated with ethanol and xylene to prep for mounting.