Clonal Selection of Antibody-Producing Cells
The clonal selection hypothesis has become a widely accepted model for how the immune system responds to infection and how certain types of B and T lymphocytes are selected for destruction of specific antigens invading the body.

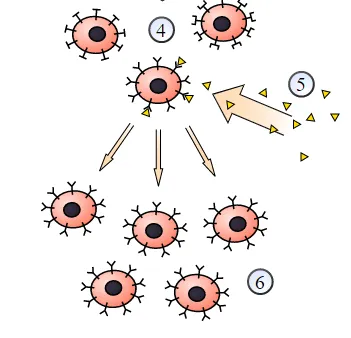
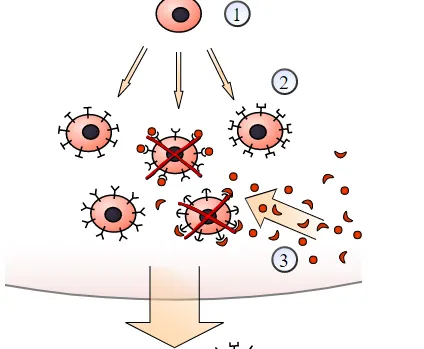
Figure: A schematic view of selection: Clonal selection of lymphocytes: 1) A hematopoietic stem cell undergoes differentiation and genetic rearrangement to produce 2) immature lymphocytes with many different antigen receptors. Those that bind to 3) antigens from the body’s own tissues are destroyed, while the rest mature into 4) inactive lymphocytes. Most of these will never encounter a matching 5) foreign antigen, but those that do are activated and produce 6) many clones of themselves.
· Each lymphocyte bears a single type of receptor with a unique specificity (by V(D)J recombination).
· Receptor occupation is required for cell activation.
· The differentiated effector cells derived from an activated lymphocyte will bear receptors of identical specificity as the parental cell.
· Those lymphocytes bearing receptors for self molecules will be deleted at an early stage.
In 1954, Danish immunologist Niels Jerne put forward a hypothesis which stated that there is already a vast array of lymphocytes in the body prior to any infection. The entrance of an antigen into the body results in the selection of only one type of lymphocyte to match it and produce a corresponding antibody to destroy the antigen. This selection of only one type of lymphocyte results in it being cloned or reproduced by the body extensively to ensure there are enough antibodies produced to inhibit and prevent infection. Australian immunologist Frank Macfarlane Burnet, with input from David W. Talmage, worked on this model and was the first to name it “clonal selection theory. ” Burnet explained immunological memory as the cloning of two types of lymphocyte. One clone acts immediately to combat infection whilst the other is longer lasting, remaining in the immune system for a long time, which results in immunity to that antigen. In 1958, Sir Gustav Nossal and Joshua Lederberg showed that one B cell always produces only one antibody, which was the first evidence for clonal selection theory.
B cells exist as clones. All B cells derive from a particular cell, and as such, the antibodies and their differentiated progenies can recognize and/or bind the same specific surface components composed of biological macromolecules ( epitope ) of a given antigen. Such clonality has important consequences, as immunogenic memory relies on it. The great diversity in immune response comes about due to the up to 109 clones with specificities for recognizing different antigens. Upon encountering its specific antigen, a single B cell, or a clone of cells with shared specificity, divides to produce many B cells. Most of such B cells differentiate into plasma cells that secrete antibodies into blood that bind the same epitope that elicited proliferation in the first place. A small minority survives as memory cells that can recognize only the same epitope. However, with each cycle, the number of surviving memory cells increases. The increase is accompanied by affinity maturation which induces the survival of B cells that bind to the particular antigen with high affinity. This subsequent amplification with improved specificity of immune response is known as secondary immune response. B cells that have not been activated by antigen are known as naive lymphocytes; those that have met their antigen, become activated, and have differentiated further into fully functional lymphocytes are known as effector B lymphocytes.
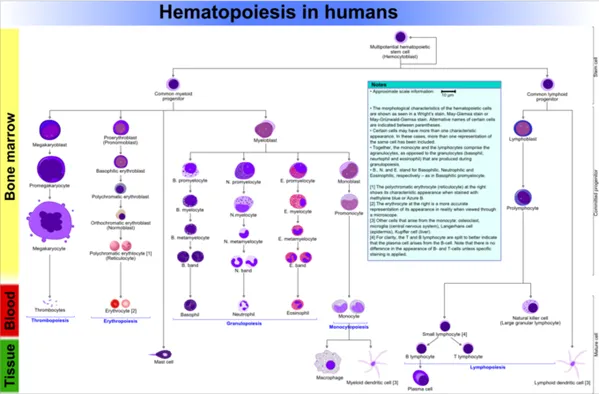
Figure: Hematopoiesis in Humans: This diagram shows hematopoiesis as it occurs in humans.