Receptor Agonists and Antagonists
Receptors are transmembrane proteins that enable the transfer of chemical signals from the cellular outer microenvironment into the cells, as well as within the cell. Receptors are usually embedded into the membrane and face either outside of the cells (extracellular receptors) or into the cytosol (cytosolic receptors). They may also reside in the nucleus of a cell (nuclear receptors or transcription factors).
Receptors allow for cell-cell communication and also the regulation of many cellular biochemical processes (transcription of genes, protein phosphorylation, activation of enzymes, etc.). The state of activation for a receptor is changed when the receptor binds to a ligand (ie. endogenous ligands may be peptides, hormones, neurotransmitters, vitamins A and D, or other compounds). Ligand binding usually triggers a conformational change in the receptor, that can either activate or deactivate the receptor, and initiate a cascade of events which are necessary to produce a biological functional effect. In some cases, the occupied receptor actually expresses biological activity itself. For example, an occupied receptor can acquire enzymatic activity, or become an active ion channel.
Xenobiotic ligands that elicit the same biological effects as the natural ligand are called "Receptor Agonists". Conversely, "Receptor Antagonists" are the ligands that inhibit the biological activity of the receptor. Note that the concept of receptor agonism and antagonism only refers to the interaction between receptors and ligands and not to their biological effects.
Receptor Agonists
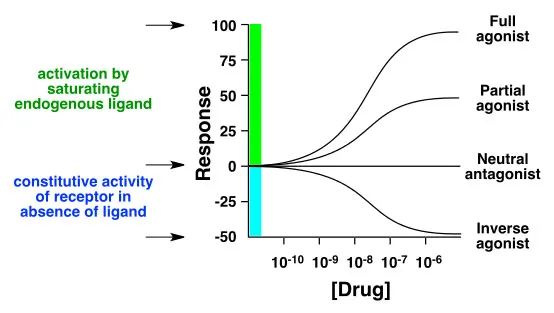
An agonist is a mimetic of the natural ligand and produces a similar biological effect as the natural ligand when it binds to the receptor. Agonists bind to the receptor at the same binding site as the natural ligand, and results in either a full (conventional agonists) or partial (partial agonists) activation. Conventional agonists increase the proportion of activated receptors. Inverse agonists are agents that bind to the same receptor as an agonist but induces a pharmacological response opposite to that agonist. The term "inverse agonist" response makes sense only whenthe un-bound, free receptor has a basal, or constitutive activity.
A conventional agonist increases the activity of a receptor above its basal level, whereas an inverse agonist decreases the activity below the basal level. An example of a receptor that possesses basal activity and for which inverse agonists have been identified is the GABA receptor. Agonists of the GABA receptor (such as benzodiazepines) create a sedative effect, whereas inverse agonists (for example, Ro15-4513) have an anxiogenic effect, or even a convulsive effect (certain beta-carbolines).
A neutral antagonist has no activity in the absence of an agonist or inverse agonist but can block the activity of either. The efficacy of a full agonist is by definition 100%, a neutral antagonist has 0% efficacy, and an inverse agonist has < 0% (ie. negative) efficacy (see graph below).

Receptor Antagonists
Receptor antagonists are inhibitors of receptor activity. Antagonists mimic ligands that bind to a receptor and prevent receptor activation by a natural ligand. Preventing activation may have many effects. If a natural agonist binding to a receptor leads to an increase in cellular function, an antagonist that binds and blocks this receptor decreases the function. Antagonists increase cellular function if they block the action of a substance that normally decreases cellular function. Conversely, antagonists decrease cellular function if they block the action of a substance that normally increases cellular function. Receptor antagonists can be classified as reversible or irreversible. Reversible antagonists readily dissociate from their receptor; irreversible antagonists form a stable chemical bond with their receptor (e.g., by alkylation). Pseudo-irreversible antagonists slowly dissociate from their receptor over time. Three types of receptor antagonists are described: competitive (binding to active site of a receptor), non-competitive (binding to a different site than the active site, or theallosteric site of the receptor) and uncompetitive. Uncompetitive antagonists differ from non-competitive antagonists in that they require receptor activation by an agonist before they can bind to a separate allosteric binding site.