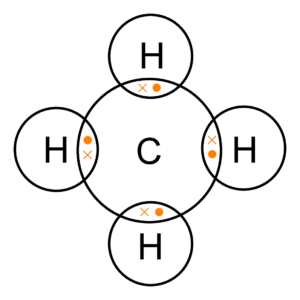
Covalent Bond
Covalent bond is a chemical bonding process in which pairs of electrons are shared between two atoms. The force of attraction or repulsion between two atoms, when they share electron pair or bonding pairs, is called as Covalent Bonding. Carbon, having four electrons in its outer shell has given it the ability to form innumerable molecules and bonds. This is why carbon has so many elements and allotropes. Confused as to why? It is because a carbon atom is in the most favorable situation to form a covalent bond. Let us learn further.
Covalent bonding occurs between non-metal elements when pairs of electrons are shared by atoms. Atoms will covalently bond with other atoms to attain the nearest noble gas configuration. Here when elements share their electrons, they do not become positive or negative, since they are neither gaining or sacrificing compounds. Thus no ions are formed by covalent bonding.
Let us learn about covalent bonding through examples given below
The simplest way to learn about covalent bond is the example of a hydrogen molecule. Are you aware that hydrogen that is present in our atmosphere cannot exist in its original form? It has to bond with another atom, for it to be stable enough. This is why the molecular formula of hydrogen is always H2.
A single atom of Hydrogen has one electron, i.e. its atomic number 1. It has its only electron in its first and only orbit. Now to be a stable molecule it needs to complete its duplet state. So a single hydrogen atom will remain unstable unit it attains one more electron. So we can say the valency of hydrogen is 1. The valency of an atom depends on its sharing capacity. So the hydrogen atom shares its single atom with another hydrogen atom. Now both hydrogen atoms have two (shared) electrons in its outer shell and it is a stable molecule H2. This bond formed by sharing electrons is nothing but a covalent bond.
· Carbon
· Versatile Nature of Carbon
· Some Important Carbon Compounds
· Chemical Properties of Carbon Compounds
Covalent carbon compounds are those where there is a carbon-carbon bond. These covalent compounds have stronger bonds than other compounds. This is because carbon is a small atom. Its nucleus has a strong force of attraction and holds these bonds tightly together. So covalent carbon compounds have a strong bond between themselves. Now let us understand why the covalent bonding is so relevant for carbon atoms.
As you are aware the reactivity of elements is its ability to lose or gain electrons from so that its outermost shell has a complete octet (or duplet in case of hydrogen) namely attain noble gas configuration. However, carbon has a unique situation. It has four electrons in its outermost shell, so one of the following situations must happen.
· It can loose the four electrons in its last shell and become a cation i.e. C4+. However to lose all four of these electrons would require a large amount of energy, and the resulting atom would be unstable with six neutrons holding only two electrons in one shell.
· The other option a carbon atom has is to obviously gain four electrons from another atom. But it would be extremely difficult for the resulting carbon atom to be stable. Ten electrons will have to be held by six neutrons in the nucleus.
So instead, carbon comes up with a unique solution. It shares its valence electrons with those of other carbon atoms, or even atoms of other elements. Now these shared atoms of the last shell, belong to both the atoms, hence forming a bond between these atoms. Now both atoms have a complete outer shell with eight atoms and have both attained noble gas configuration. This sharing of atoms, instead of gaining or losing is called covalent bonding. And Carbon, since its atomic number is 6, and it has four electrons in the last shell has the most favourable structure for covalent bonding.
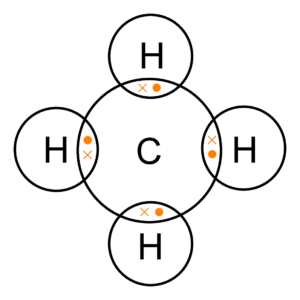
Methane: Now let us take a look at covalent bonding in some carbon elements. Let’s get started with that of Methane. Its chemical formula is CH4. Now that means one atom of carbon combines with four atoms of Hydrogen to make one molecule of methane. All the hydrogen atoms have only one electron in their outermost shell and carbon has four. So carbon shares each of its four electrons with one atom of carbon. This way carbon now has a complete octet and all four hydrogen atoms have a complete duplet.
Carbon dioxide: Let us take a look at another element you should be very familiar with, carbon dioxide. Here two atoms of oxygen combine with one atom of carbon thus giving us the molecule CO2. As we already know now that carbon has four electrons in its outer shell, whereas oxygen with atomic number 8, has six atoms in the last shell. So carbon shares two of its atoms with each atom of oxygen. This way all three atoms complete their octet giving us one stable molecule.