
Kinetic Isotope Effects
The kinetic isotope effect (KIE) is a phenomenon associated with isotopically substituted molecules exhibiting different reaction rates. Isotope effects such as KIEs are invaluable tools in both physical and biological sciences and are used to aid in the understanding of reaction kinetics, mechanisms, and solvent effects.
Research was first introduced on this topic over 50 years ago and has grown into an enormous field. The scientists behind much of the understanding and development of kinetic isotope effects were Jacob Bigeleisen and Maria Goeppert Mayer who published the first paper on isotope effects [J. Chem. Phys., 15, 261 (1947)]. Kinetic isotope effects specifically explore the change in rate of a reaction due to isotopic substitution.
An element is identified by its symbol, mass number, and atomic number. The atomic number is the number of protons in the nucleus while the mass number is the total number of protons and neutrons in the nucleus. Isotopes are two atoms of the same element that have the same number of protons but different numbers of neutrons. Isotopes are specified by the mass number.

As an example consider the two isotopes of chlorine, you can see that their mass numbers vary, with 35Cl being the most abundant isotope, while their atomic numbers remain the same at 17.


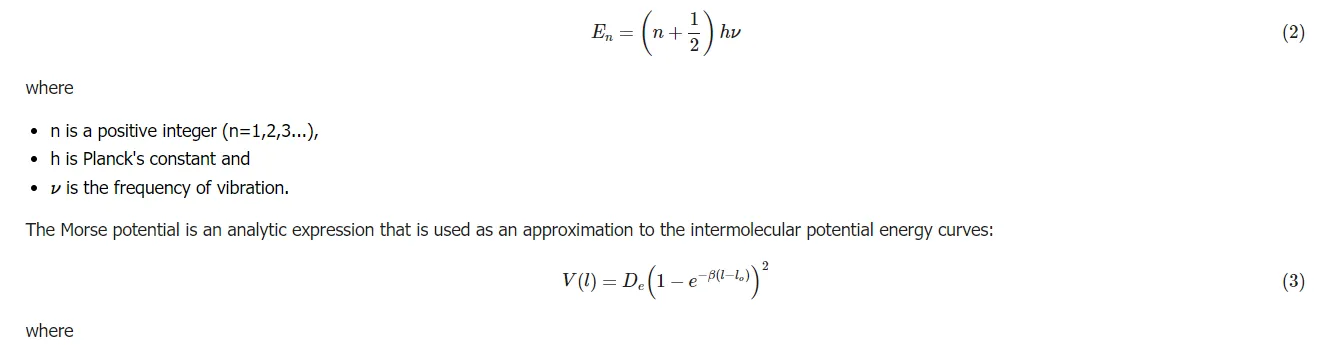
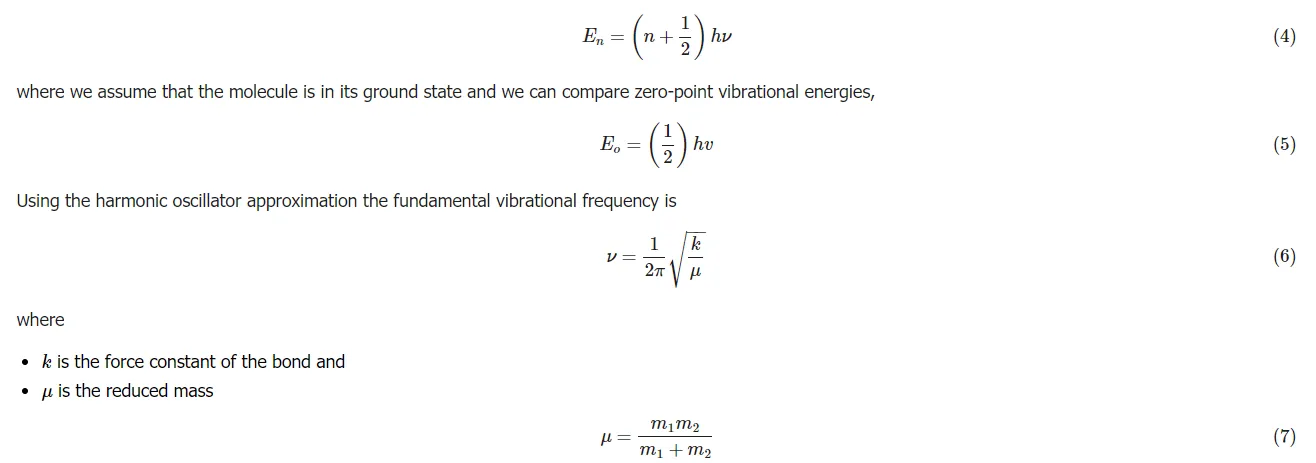
Understanding potential energy surfaces is important in order to be able to understand why and how isotope effects occur as they do. The harmonic oscillator approximation is used to explain the vibrations of a diatomic molecule. The energies resulting from the quantum mechanic solution for the harmonic oscillator help to define the internuclear potential energy of a diatomic molecule and are

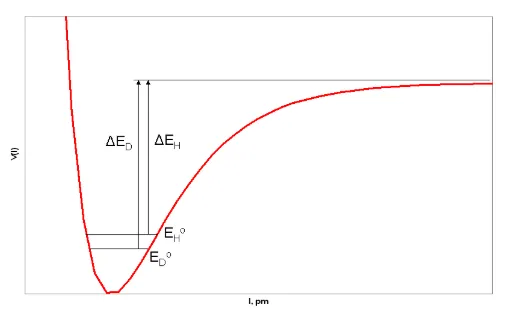
This results in different bond dissociation energies for R-D and R-H. The bond dissociation energy for R-D (ED) is greater than the bond dissociation energy of R-H (EH). This difference in energy due to isotopic replacement results in differing rates of reaction, the effect that is measured in kinetic isotope effects. The reaction rate for the conversion of R-D is slower than the reaction rate for the conversion of R-H.
 p>
p>
It is important to note that isotope replacement does not change the electronic structure of the molecule or the potential energy surfaces of the reactions the molecule may undergo. Only the rate of the reaction is affected.
The energy of the vibrational levels of a vibration (i.e., a bond) in a molecule is given by
The Arrhenius equation is used to determine reaction rates and activation energies and since we are interested in the change in rate of reactions with different isotopes, this equation is very important,
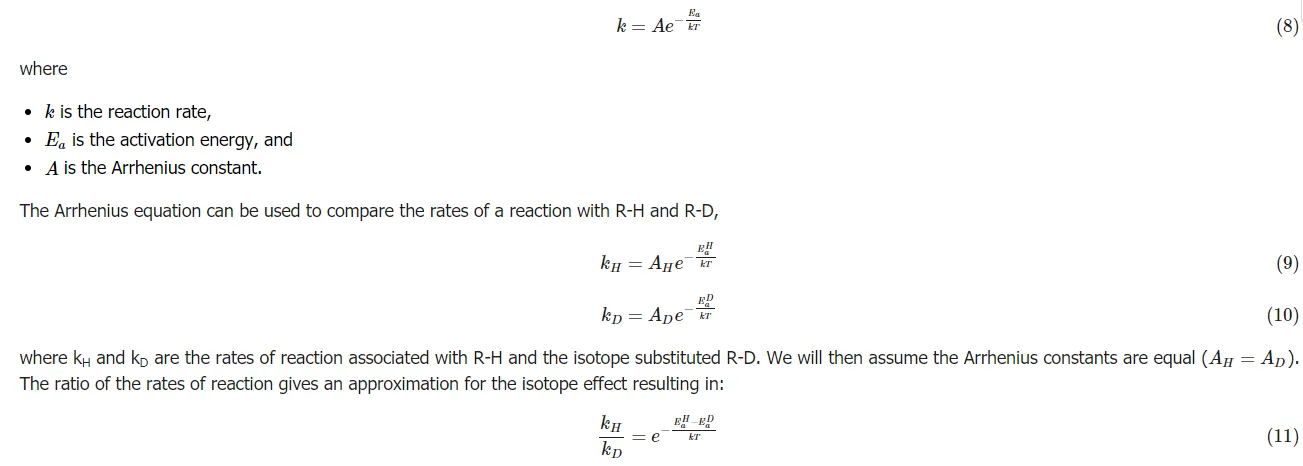
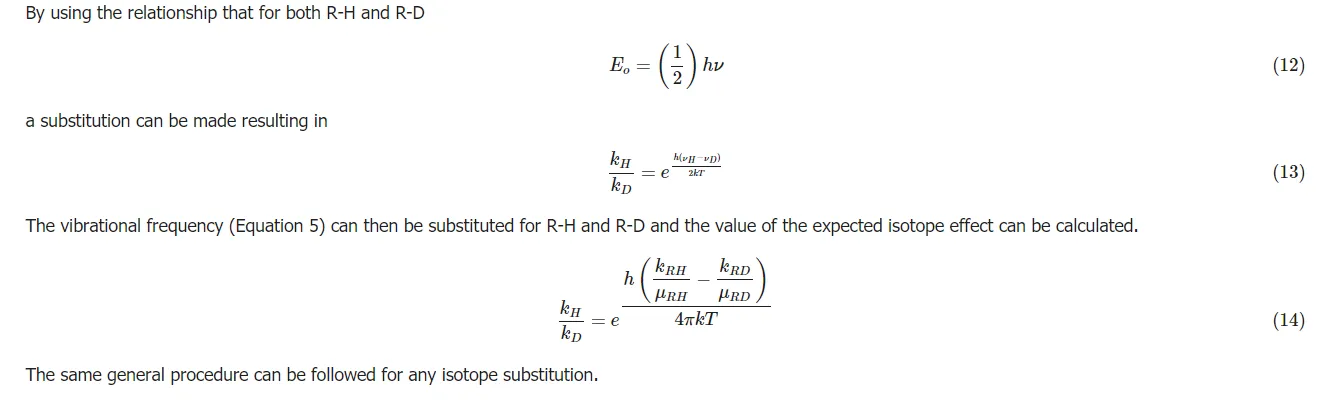
The same general procedure can be followed for any isotope substitution.
In summary, the greater the mass the more energy is needed to break bonds. A heavier isotope forms a stronger bond. The resulting molecule has less of a tendency to dissociate. The increase in energy needed to break the bond results in a slower reaction rate and the observed isotope effect.
Kinetic Isotope Effects (KIEs) are used to determine reaction mechanisms by determining rate limiting steps and transition states and are commonly measured using NMR to detect isotope location or GC/MS to detect mass changes. In a KIE experiment an atom is replaced by its isotope and the change in rate of the reaction is observed. A very common isotope substitution is when hydrogen is replaced by deuterium. This is known as a deuterium effect and is expressed by the ratio kH/kD (as explained above). Normal KIEs for the deuterium effect are around 1 to 7 or 8. Large effects are seen because the percentage mass change between hydrogen and deuterium is great. Heavy atom isotope effects involve the substitution of carbon, oxygen, nitrogen, sulfur, and bromine, with effects that are much smaller and are usually between 1.02 and 1.10. The difference in KIE magnitude is directly related to the percentage change in mass. Large effects are seen when hydrogen is replaced with deuterium because the percentage mass change is very large (mass is being doubled) while smaller percent mass changes are present when an atom like sulfur is replaced with its isotope (increased by two mass units).