Kinetic Versus Thermodynamic Products
Kinetic products form the fastest. They usually occur at or below 0°C. This is also known as the 1,2-adduct because the substituents are added to the first and second carbons. Kinetic products contain a terminal double bond and the reaction is irreversible. Thermodynamic products form at higher temperatures, generally greater than 40 °C. These are known are the 1,4-adducts because they add to the first and fourth carbons. Thermodynamic products contain an internal double bond and the reaction is reversible. Also, when reactions are carried out, thermodynamic products are more stable than kinetic products because they are more substituted.
Final Products:
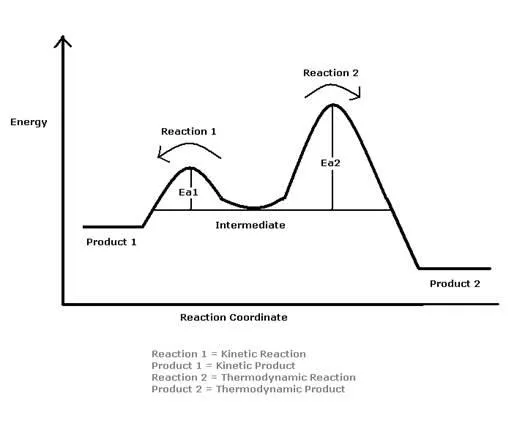
The reason for the two products is the difference in their activation energies. The reaction will go through to completion on the easiest path and in this case that is the Kinetic path. This path has a much lower Activation Energy which means that less is required to have the product formed. This product- Product A, is not the most stable form however. Over time, the amount of Product B- the more stable one, will increase and the Product A will decrease.
To ensure the greatest possible yield of thermodynamic products, the reaction should be carried out at a temperature of 40°C or greater. This is known as thermodynamic control. At higher temperatures and longer reaction times, thermodynamic products are favored. On the contrary, at lower temperatures, one would tend to see a greater yield of kinetic products. These products are generally formed at or around 0°C. Carrying out reactions around these temperatures is known as kinetic control and kinetic products form before thermodynamic products.

Since the thermodynamic product contains an internal double bond, it is more stable than the kinetic product, and this is due to hyperconjugation with neighboring atoms. Additionally, a higher activation energy results in the thermodynamic product forming slower than the kinetic product. Therefore, a thermodynamically controlled reaction gives a more stable product and kinetically controlled reaction gives a less stable product.

The conjugated diene has 2 double bonds with one single C-C bond between them. This structure offers stability because the two pi bonds can transfer electrons through the two carbons that are sp2 hybridized with a single bond which results in electron delocalization. Extended P orbital sharing makes this diene more stable than the isolated dienes. The more stable molecule also has lower energy and a shorter bond length.
The p electrons reach out to the electrophile and form a bond that in turn forms a Carbocation. The Markovnikov Addition states that the most stable carbocation is most likely to be formed with the charge going on the more substituted carbon. The difference between a conjugated diene and an alkene is that there is still a double bond left after the reaction has completed.