Acid-Catalyzed Ester Hydrolysis
Water the Destroyer
Imagine that water, the stuff you drink everyday, is actually a superhero with a cape, bulging muscles, and a samurai sword. Let's call him 'Water the Destroyer' (complete with a thunderclap every time his name is uttered). And since every superhero needs his go-to move, Water the Destroyer's super move is called hydrolysis. The actual definition of hydrolysis, the breakdown of a compound due to a reaction with water, actually fits very well with the superhero analogy!
But if water is the superhero, then who is the super-villain? Typically, when hydrolysis is mentioned, the compound being broken down is written just beforehand, such as ester hydrolysis. So in this case, our super-villain, the compound being destroyed, is an ester. So writing 'ester hydrolysis' is sort of like saying 'Water the Destroyer hydrolyzed Ester in the face!' And after being defeated, the ester becomes a law-abiding carboxylic acid and alcohol.
Our superhero, however, cannot defeat the super-villain alone. He needs one of his trusty side-kicks, or catalysts (in this case, acid). A catalyst is a substance that makes a reaction happen, or happen faster, without changing itself - sort of like how a side-kick helps in the overall victory, but is not the one fighting the majority of the battle. So when we write 'acid-catalyzed ester hydrolysis', we are saying, 'Acid helped Water the Destroyer hydrolyze Ester in the face!'
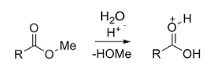
In short, an acid-catalyzed ester hydrolysis breaks down an ester into a carboxylic acid and an alcohol, using an acid as a catalyst. You can see this in the example below. The methyl ester, when exposed to an acid, is broken down into methyl alcohol (seen written below the arrow), and the resulting carboxylic acid (shown above the arrow with a H+ ion attached since it is in acidic solution).
|
Procedure
When performing an acid-catalyzed ester hydrolysis the ester is combined with a diluted acid solution, often hydrochloric acid or sulfuric acid. The combined solution is then heated at reflux, which simply means boiling the solution with a condenser attached so none of the mixture is lost. Since the reaction is reversible, it is important to overload the reaction with reactants in order to push the reaction forward. The acid, being in an aqueous solution, makes water abundant, accomplishing this overload of reactants nicely.
Mechanism
|
The first step in the reaction mechanism is the donation of a proton, or H+ ion, by the acid, which naturally gravitates to the most nucleophilic site on the ester. A nucleophile is simply a site with extra electrons, which are negatively charged, that seeks a positively charged counterpart, such as the H+ ion.
After the ester has been protonated, or had the H+ ion added, the carbon in the carbonyl group becomes much more electrophilic (seeks electron-rich areas), and therefore can more readily accept the electrons of a water molecule.