Optical Activity
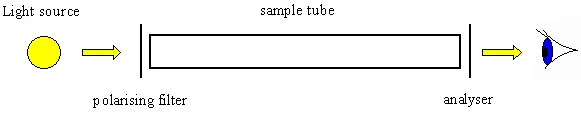
Optical activity is the ability of a chiral molecule to rotate the plane of plane-polairsed light, measured using a polarimeter. A simple polarimeter consists of a light source, polarising lens, sample tube and analysing lens.

When light passes through a sample that can rotate plane polarised light, the light appears to dim because it no longer passes straight through the polarising filters. The amount of rotation is quantified as the number of degrees that the analysing lens must be rotated by so that it appears as if no dimming of the light has occurred.
When rotation is quantified using a polarimeter it is known as an observed rotation, because rotation is affected by path length (l, the time the light travels through a sample) and concentration (c, how much of the sample is present that will rotate the light). When these effects are eliminated a standard for comparison of all molecules is obtained, the specific rotation, [a].
[a] = 100a / cl when concentration is expressed as g sample /100ml solution
Specific rotation is a physical property like the boiling point of a sample and can be looked up in reference texts. Take a look at a problem.
Enantiomers will rotate the plane of polarisation in exactly equal amounts (same magnitude) but in opposite directions.
Dextrorotary designated as d or (+), clockwise rotation (to the right)
Levorotary designated as l or (-), anti-clockwise rotation (to the left)
If only one enantiomer is present a sample is considered to be optically pure. When a sample consists of a mixture of enantiomers, the effect of each enantiomer cancels out, molecule for molecule.
For example, a 50:50 mixture of two enantiomers or a racemic mixture will not rotate plane polarised light and is optically inactive. A mixture that contains one enantiomer excess, however, will display a net plane of polarisation in the direction characteristic of the enantiomer that is in excess.
The optical purity or the enantiomeric excess (ee%) of a sample can be determined as follows:
Optical purity = % enantiomeric excess = % enantiomer1 - % enantiomer2
= 100 [a]mixture / [a]pure sample
ee% = 100 ([major enantiomer] - [minor enantiomer]) / ([major enantiomer] + [minor enantiomer])
where [major enantiomer] = concentration of the major enantiomer
[minor enantiomer] = concentration of the minor enantiomer
Look at some problems like these more in depth.
Diasteromeric substances can have different rotations both in sign and in magnitude.