Properties of Lipids
These organic compounds are nonpolar molecules, which are soluble only in nonpolar solvents and insoluble in water because water is polar molecules. In the human body, these molecules can be synthesized in the liver and are and generally found in the oil, butter, whole milk, cheese, fried foods, and also in some red meats.
Let us have a detailed look at the lipid structure, properties, types and classification of lipids.

Lipids are a family of organic compounds, composed of fats and oils. These molecules yield high energy and are responsible for different functions within the human body. Listed below are some important characteristics of Lipids.
Also Read: Digestion and Absorption of Lipids
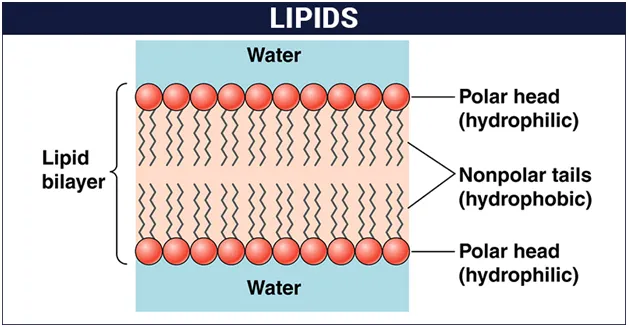
Lipids are the polymers of fatty acids that contain a long, non-polar hydrocarbon chain with a small polar region containing oxygen. The lipid structure is explained in the diagram below:
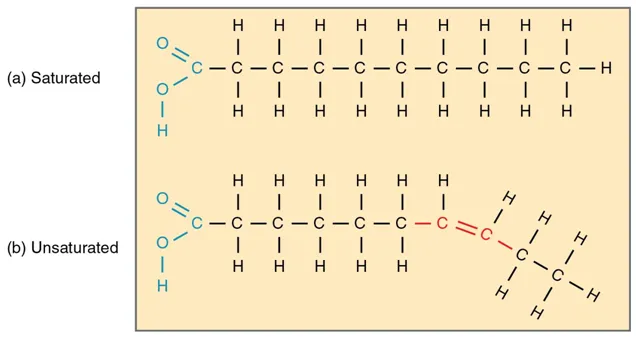
Lipid Structure – Saturated and Unsaturated Fatty Acids
Lipids can be classified into two major classes:
A nonsaponifiable lipid cannot be broken up into smaller molecules by hydrolysis, which includes triglycerides, waxes, phospholipids, and sphingolipids.
A saponifiable lipid contains one or more ester groups allowing it to undergo hydrolysis in the presence of an acid, base, or enzymes. Nonsaponifiable lipids include steroids, prostaglandins, and terpenes.
Each of these categories can be further broken down into non-polar and polar lipids.
Nonpolar lipids, such as triglycerides, are used for energy storage and fuel.
Polar lipids, which can form a barrier with an external water environment, are used in membranes. Polar lipids include glycerophospholipids and sphingolipids.
Fatty acids are important components of all of these lipids.