Prostaglandins
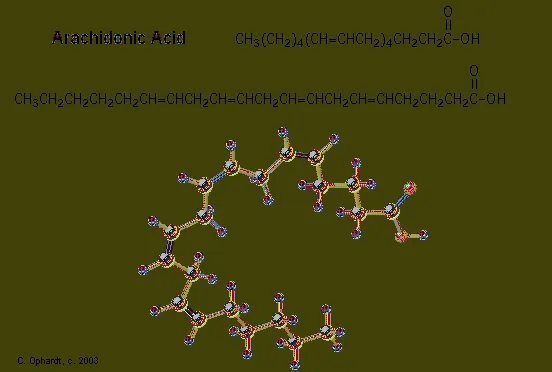
Prostaglandins are unsaturated carboxylic acids, consisting of of a 20 carbon skeleton that also contains a five member ring. They are biochemically synthesized from the fatty acid, arachidonic acid. See the graphic below. The unique shape of the arachidonic acid caused by a series of cis double bonds helps to put it into position to make the five member ring of the prostaglandin.

Prostaglandin Structure
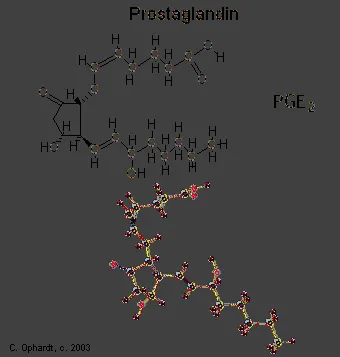
Prostaglandins are unsaturated carboxylic acids, consisting of of a 20 carbon skeleton that also contains a five member ring and are based upon the fatty acid, arachidonic acid. There are a variety of structures one, two, or three double bonds. On the five member ring there may also be double bonds, a ketone, or alcohol groups. A typical structure is shown below.
Functions of Prostaglandins
There are a variety of physiological effects including:
Activation of the inflammatory response, production of pain, and fever. When tissues are damaged, white blood cells flood to the site to try to minimize tissue destruction. Prostaglandins are produced as a result.
Blood clots form when a blood vessel is damaged. A type of prostaglandin called thromboxane stimulates constriction and clotting of platelets. Conversely, PGI2, is produced to have the opposite effect on the walls of blood vessels where clots should not be forming.
Certain prostaglandins are involved with the induction of labor and other reproductive processes. PGE2 causes uterine contractions and has been used to induce labor.
Prostaglandins are involved in several other organs such as the gastrointestinal tract (inhibit acid synthesis and increase secretion of protective mucus), increase blood flow in kidneys, and leukotriens promote constriction of bronchi associated with asthma.
Effects of Aspirin and other Pain Killers
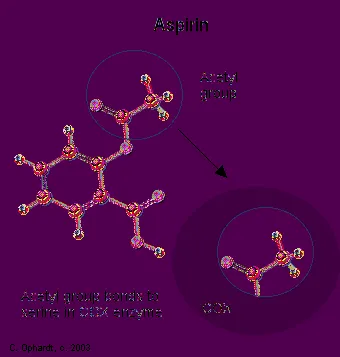
When you see that prostaglandins induce inflammation, pain, and fever, what comes to mind but aspirin. Aspirin blocks an enzyme called cyclooxygenase, COX-1 and COX-2, which is involved with the ring closure and addition of oxygen to arachidonic acid converting to prostaglandins. The acetyl group on aspirin is hydrolzed and then bonded to the alcohol group of serine as an ester. This has the effect of blocking the channel in the enzyme and arachidonic can not enter the active site of the enzyme. By inhibiting or blocking this enzyme, the synthesis of prostaglandins is blocked, which in turn relives some of the effects of pain and fever. Aspirin is also thought to inhibit the prostaglandin synthesis involved with unwanted blood clotting in coronary heart disease. At the same time an injury while taking aspirin may cause more extensive bleeding.
Eicosanoids
Prostaglandins are one example of biologically important class of fatty acids called eicosanoids. Derived primarily from arachidonic acid (5,8,11,14-eicosatetraenoic acid), eicosanoids include prostaglandins, leukotrienes, and thromboxanes.

The members of this group of structurally related natural hormones have an extraordinary range of biological effects. They can lower gastric secretions, stimulate uterine contractions, lower blood pressure, influence blood clotting and induce asthma-like allergic responses. Because their genesis in body tissues is tied to the metabolism of the essential fatty acid arachadonic acid (5,8,11,14-eicosatetraenoic acid) they are classified as eicosanoids. Many properties of the common drug aspirin result from its effect on the cascade of reactions associated with these hormones.
The metabolic pathways by which arachidonic acid is converted to the various eicosanoids are complex and will not be discussed here. A rough outline of some of the transformations that take place is provided below. It is helpful to view arachadonic acid in the coiled conformation shown in the shaded box.
