Sliding Filament Model of Contraction
For a muscle cell to contract, the sarcomere must shorten. However, thick and thin filaments—the components of sarcomeres—do not shorten. Instead, they slide by one another, causing the sarcomere to shorten while the filaments remain the same length. The sliding filament theory of muscle contraction was developed to fit the differences observed in the named bands on the sarcomere at different degrees of muscle contraction and relaxation. The mechanism of contraction is the binding of myosin to actin, forming cross-bridges that generate filament movement (Figure 19.36).
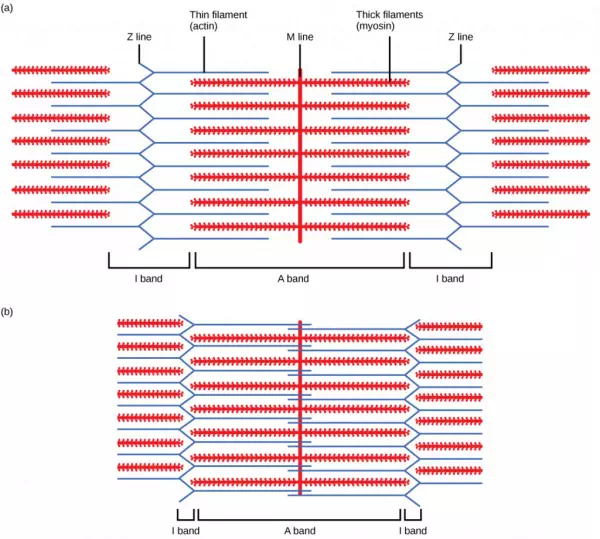
Figure 19.36. When (a) a sarcomere (b) contracts, the Z lines move closer together and the I band gets smaller. The A band stays the same width and, at full contraction, the thin filaments overlap.
When a sarcomere shortens, some regions shorten whereas others stay the same length. A sarcomere is defined as the distance between two consecutive Z discs or Z lines; when a muscle contracts, the distance between the Z discs is reduced. The H zone—the central region of the A zone—contains only thick filaments and is shortened during contraction. The I band contains only thin filaments and also shortens. The A band does not shorten—it remains the same length—but A bands of different sarcomeres move closer together during contraction, eventually disappearing. Thin filaments are pulled by the thick filaments toward the center of the sarcomere until the Z discs approach the thick filaments. The zone of overlap, in which thin filaments and thick filaments occupy the same area, increases as the thin filaments move inward.
ATP and Muscle Contraction
The motion of muscle shortening occurs as myosin heads bind to actin and pull the actin inwards. This action requires energy, which is provided by ATP. Myosin binds to actin at a binding site on the globular actin protein. Myosin has another binding site for ATP at which enzymatic activity hydrolyzes ATP to ADP, releasing an inorganic phosphate molecule and energy.
ATP binding causes myosin to release actin, allowing actin and myosin to detach from each other. After this happens, the newly bound ATP is converted to ADP and inorganic phosphate, Pi. The enzyme at the binding site on myosin is called ATPase. The energy released during ATP hydrolysis changes the angle of the myosin head into a “cocked” position. The myosin head is then in a position for further movement, possessing potential energy, but ADP and Pi are still attached. If actin binding sites are covered and unavailable, the myosin will remain in the high energy configuration with ATP hydrolyzed, but still attached.
If the actin binding sites are uncovered, a cross-bridge will form; that is, the myosin head spans the distance between the actin and myosin molecules. Pi is then released, allowing myosin to expend the stored energy as a conformational change. The myosin head moves toward the M line, pulling the actin along with it. As the actin is pulled, the filaments move approximately 10 nm toward the M line. This movement is called the power stroke, as it is the step at which force is produced. As the actin is pulled toward the M line, the sarcomere shortens and the muscle contracts.
When the myosin head is “cocked,” it contains energy and is in a high-energy configuration. This energy is expended as the myosin head moves through the power stroke; at the end of the power stroke, the myosin head is in a low-energy position. After the power stroke, ADP is released; however, the cross-bridge formed is still in place, and actin and myosin are bound together. ATP can then attach to myosin, which allows the cross-bridge cycle to start again and further muscle contraction can occur (Figure 19.37).
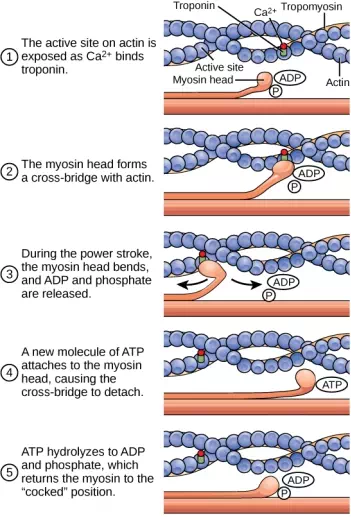
Figure 19.37. The cross-bridge muscle contraction cycle, which is triggered by Ca2+ binding to the actin active site, is shown. With each contraction cycle, actin moves relative to myosin.
Which of the following statements about muscle contraction is true?
1. The power stroke occurs when ATP is hydrolyzed to ADP and phosphate.
2. The power stroke occurs when ADP and phosphate dissociate from the myosin head.
3. The power stroke occurs when ADP and phosphate dissociate from the actin active site.
4. The power stroke occurs when Ca2+ binds the calcium head.
Regulatory Proteins
When a muscle is in a resting state, actin and myosin are separated. To keep actin from binding to the active site on myosin, regulatory proteins block the molecular binding sites. Tropomyosinblocks myosin binding sites on actin molecules, preventing cross-bridge formation and preventing contraction in a muscle without nervous input. Troponin binds to tropomyosin and helps to position it on the actin molecule; it also binds calcium ions.
To enable a muscle contraction, tropomyosin must change conformation, uncovering the myosin-binding site on an actin molecule and allowing cross-bridge formation. This can only happen in the presence of calcium, which is kept at extremely low concentrations in the sarcoplasm. If present, calcium ions bind to troponin, causing conformational changes in troponin that allow tropomyosin to move away from the myosin binding sites on actin. Once the tropomyosin is removed, a cross-bridge can form between actin and myosin, triggering contraction. Cross-bridge cycling continues until Ca2+ ions and ATP are no longer available and tropomyosin again covers the binding sites on actin.
Excitation–Contraction Coupling
Excitation–contraction coupling is the link (transduction) between the action potential generated in the sarcolemma and the start of a muscle contraction. The trigger for calcium release from the sarcoplasmic reticulum into the sarcoplasm is a neural signal. Each skeletal muscle fiber is controlled by a motor neuron, which conducts signals from the brain or spinal cord to the muscle. The area of the sarcolemma on the muscle fiber that interacts with the neuron is called the motor end plate. The end of the neuron’s axon is called the synaptic terminal, and it does not actually contact the motor end plate. A small space called the synaptic cleft separates the synaptic terminal from the motor end plate. Electrical signals travel along the neuron’s axon, which branches through the muscle and connects to individual muscle fibers at a neuromuscular junction.
The ability of cells to communicate electrically requires that the cells expend energy to create an electrical gradient across their cell membranes. This charge gradient is carried by ions, which are differentially distributed across the membrane. Each ion exerts an electrical influence and a concentration influence. Just as milk will eventually mix with coffee without the need to stir, ions also distribute themselves evenly, if they are permitted to do so. In this case, they are not permitted to return to an evenly mixed state.
The sodium–potassium ATPase uses cellular energy to move K+ ions inside the cell and Na+ ions outside. This alone accumulates a small electrical charge, but a big concentration gradient. There is lots of K+ in the cell and lots of Na+ outside the cell. Potassium is able to leave the cell through K+channels that are open 90% of the time, and it does. However, Na+ channels are rarely open, so Na+remains outside the cell. When K+ leaves the cell, obeying its concentration gradient, that effectively leaves a negative charge behind. So at rest, there is a large concentration gradient for Na+ to enter the cell, and there is an accumulation of negative charges left behind in the cell. This is the resting membrane potential. Potential in this context means a separation of electrical charge that is capable of doing work. It is measured in volts, just like a battery. However, the transmembrane potential is considerably smaller (0.07 V); therefore, the small value is expressed as millivolts (mV) or 70 mV. Because the inside of a cell is negative compared with the outside, a minus sign signifies the excess of negative charges inside the cell, −70 mV.
If an event changes the permeability of the membrane to Na+ ions, they will enter the cell. That will change the voltage. This is an electrical event, called an action potential, that can be used as a cellular signal. Communication occurs between nerves and muscles through neurotransmitters. Neuron action potentials cause the release of neurotransmitters from the synaptic terminal into the synaptic cleft, where they can then diffuse across the synaptic cleft and bind to a receptor molecule on the motor end plate. The motor end plate possesses junctional folds—folds in the sarcolemma that create a large surface area for the neurotransmitter to bind to receptors. The receptors are actually sodium channels that open to allow the passage of Na+ into the cell when they receive neurotransmitter signal.
Acetylcholine (ACh) is a neurotransmitter released by motor neurons that binds to receptors in the motor end plate. Neurotransmitter release occurs when an action potential travels down the motor neuron’s axon, resulting in altered permeability of the synaptic terminal membrane and an influx of calcium. The Ca2+ ions allow synaptic vesicles to move to and bind with the presynaptic membrane (on the neuron), and release neurotransmitter from the vesicles into the synaptic cleft. Once released by the synaptic terminal, ACh diffuses across the synaptic cleft to the motor end plate, where it binds with ACh receptors. As a neurotransmitter binds, these ion channels open, and Na+ions cross the membrane into the muscle cell. This reduces the voltage difference between the inside and outside of the cell, which is called depolarization. As ACh binds at the motor end plate, this depolarization is called an end-plate potential. The depolarization then spreads along the sarcolemma, creating an action potential as sodium channels adjacent to the initial depolarization site sense the change in voltage and open. The action potential moves across the entire cell, creating a wave of depolarization.
ACh is broken down by the enzyme acetylcholinesterase (AChE) into acetyl and choline. AChE resides in the synaptic cleft, breaking down ACh so that it does not remain bound to ACh receptors, which would cause unwanted extended muscle contraction (Figure 19.38).
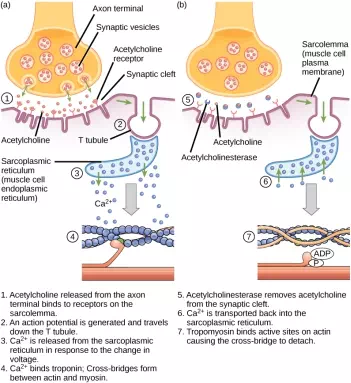
Figure 19.38. This diagram shows excitation-contraction coupling in a skeletal muscle contraction. The sarcoplasmic reticulum is a specialized endoplasmic reticulum found in muscle cells.
The deadly nerve gas Sarin irreversibly inhibits acetycholinesterase. What effect would Sarin have on muscle contraction?
After depolarization, the membrane returns to its resting state. This is called repolarization, during which voltage-gated sodium channels close. Potassium channels continue at 90% conductance. Because the plasma membrane sodium–potassium ATPase always transports ions, the resting state (negatively charged inside relative to the outside) is restored. The period immediately following the transmission of an impulse in a nerve or muscle, in which a neuron or muscle cell regains its ability to transmit another impulse, is called the refractory period. During the refractory period, the membrane cannot generate another action potential. . The refractory period allows the voltage-sensitive ion channels to return to their resting configurations. The sodium potassium ATPase continually moves Na+ back out of the cell and K+ back into the cell, and the K+ leaks out leaving negative charge behind. Very quickly, the membrane repolarizes, so that it can again be depolarized.
Control of Muscle Tension
Neural control initiates the formation of actin–myosin cross-bridges, leading to the sarcomere shortening involved in muscle contraction. These contractions extend from the muscle fiber through connective tissue to pull on bones, causing skeletal movement. The pull exerted by a muscle is called tension, and the amount of force created by this tension can vary. This enables the same muscles to move very light objects and very heavy objects. In individual muscle fibers, the amount of tension produced depends on the cross-sectional area of the muscle fiber and the frequency of neural stimulation.
The number of cross-bridges formed between actin and myosin determine the amount of tension that a muscle fiber can produce. Cross-bridges can only form where thick and thin filaments overlap, allowing myosin to bind to actin. If more cross-bridges are formed, more myosin will pull on actin, and more tension will be produced.
The ideal length of a sarcomere during production of maximal tension occurs when thick and thin filaments overlap to the greatest degree. If a sarcomere at rest is stretched past an ideal resting length, thick and thin filaments do not overlap to the greatest degree, and fewer cross-bridges can form. This results in fewer myosin heads pulling on actin, and less tension is produced. As a sarcomere is shortened, the zone of overlap is reduced as the thin filaments reach the H zone, which is composed of myosin tails. Because it is myosin heads that form cross-bridges, actin will not bind to myosin in this zone, reducing the tension produced by this myofiber. If the sarcomere is shortened even more, thin filaments begin to overlap with each other—reducing cross-bridge formation even further, and producing even less tension. Conversely, if the sarcomere is stretched to the point at which thick and thin filaments do not overlap at all, no cross-bridges are formed and no tension is produced. This amount of stretching does not usually occur because accessory proteins, internal sensory nerves, and connective tissue oppose extreme stretching.
The primary variable determining force production is the number of myofibers within the muscle that receive an action potential from the neuron that controls that fiber. When using the biceps to pick up a pencil, the motor cortex of the brain only signals a few neurons of the biceps, and only a few myofibers respond. In vertebrates, each myofiber responds fully if stimulated. When picking up a piano, the motor cortex signals all of the neurons in the biceps and every myofiber participates. This is close to the maximum force the muscle can produce. As mentioned above, increasing the frequency of action potentials (the number of signals per second) can increase the force a bit more, because the tropomyosin is flooded with calcium.
Summary
The body contains three types of muscle tissue: skeletal muscle, cardiac muscle, and smooth muscle. Skeleton muscle tissue is composed of sarcomeres, the functional units of muscle tissue. Muscle contraction occurs when sarcomeres shorten, as thick and thin filaments slide past each other, which is called the sliding filament model of muscle contraction. ATP provides the energy for cross-bridge formation and filament sliding. Regulatory proteins, such as troponin and tropomyosin, control cross-bridge formation. Excitation–contraction coupling transduces the electrical signal of the neuron, via acetylcholine, to an electrical signal on the muscle membrane, which initiates force production. The number of muscle fibers contracting determines how much force the whole muscle produces.