Differential Staining Techniques
Viewing Bacterial Cells
The microscope is a very important tool in microbiology, but there are limitations when it comes to using one to observe cells in general and bacterial cells in particular. Two of the most important concerns are resolution and contrast. Resolution is a limitation that we can’t do much about, since most bacterial cells are already near the resolution limit of most light microscopes. Contrast, however, can be improved by either using a different type of optical system, such as phase contrast or a differential interference contrast microscope, or by staining the cells (or the background) with a chromogenic dye that not only adds contrast, but gives them a color as well.
There are many different stains and staining procedures used in microbiology. Some involve a single stain and just a few steps, while others use multiple stains and a more complicated procedure. Before you can begin the staining procedure, the cells have to be mounted (smeared) and fixed onto a glass slide. A bacterial smear is simply that—a small amount of culture spread in a very thin film on the surface of the slide. To prevent the bacteria from washing away during the staining steps, the smear may be chemically or physically “fixed” to the surface of the slide. Heat fixing is an easy and efficient method, and is accomplished by passing the slide briefly through the flame of a Bunsen burner, which causes the biological material to become more or less permanently affixed to the glass surface.
Heat fixed smears are ready for staining. In a simple stain, dyes that are either attracted by charge (a cationic dye such as methylene blue or crystal violet) or repelled by charge (an anionic dye such as eosin or India ink) are added to the smear. Cationic dyes bind the bacterial cells which can be easily observed against the bright background. Anionic dyes are repelled by the cells, and therefore the cells are bright against the stained background. See Figures 1 and 2 for examples of both.
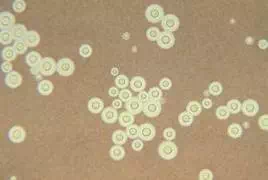
Figure 1. Negative stain of Cyptococcus neoformans, an encapsulated yeast
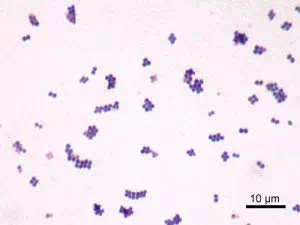
Figure 2. Positive stain of Staphylococcus aureus.
Probably the most important feature made obvious when you stain bacterial cells is their cellular morphology (not to be confused with colonial morphology, which is the appearance of bacterial colonies on an agar plate). Most heterotrophic and culturable bacteria come in a few basic shapes: spherical cells (coccus/cocci), rod-shaped cells (bacillus/bacilli), or rod-shaped cells with bends or twists (vibrios and spirilla, respectively). There is greater diversity of shapes among Archaea and other bacteria found in ecosystems other than the human body.
Often bacteria create specific arrangements of cells, which form as a result of binary fission by the bacteria as they reproduce. Arrangements are particularly obvious with non-motile bacteria, because the cells tend to stay together after the fission process is complete. Both the shape and arrangement of cells are characteristics that can be used to distinguish among bacteria. The most commonly encountered bacterial shapes (cocci and bacilli) and their possible arrangements are shown in Figures 3 and 4.
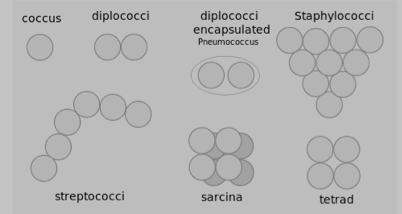
Figure 3. Possible bacterial cell arrangements for cocci
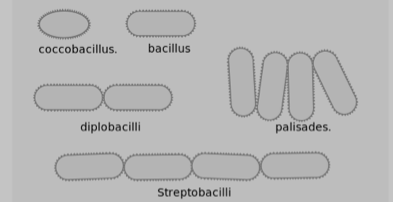
Figure 4. Possible bacteria cell arrangements for bacilli
Differential Staining Techniques
In microbiology, differential staining techniques are used more often than simple stains as a means of gathering information about bacteria. Differential staining methods, which typically require more than one stain and several steps, are referred to as such because they permit the differentiation of cell types or cell structures. The most important of these is the Gram stain. Other differential staining methods include the endospore stain (to identify endospore-forming bacteria), the acid-fast stain (to discriminate Mycobacterium species from other bacteria), a metachromatic stain to identify phosphate storage granules, and the capsule stain (to identify encapsulated bacteria). We will be performing the Gram stain and endospore staining procedures in lab, and view prepared slides that highlight some of the other cellular structures present in some bacteria.
Gram Stain
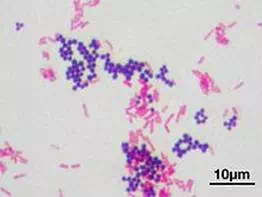
Figure 5. Bacteria stained with Gram stain.
In 1884, physician Hans Christian Gram was studying the etiology (cause) of respiratory diseases such as pneumonia. He developed a staining procedure that allowed him to identify a bacterium in lung tissue taken from deceased patients as the etiologic agent of a fatal type of pneumonia. Although it did little in the way of treatment for the disease, the Gram stain method made it much easier to diagnose the cause of a person’s death at autopsy. Today we use Gram’s staining techniques to aid in the identification of bacteria, beginning with a preliminary classification into one of two groups: Gram positive or Gram negative.
The differential nature of the Gram stain is based on the ability of some bacterial cells to retain a primary stain (crystal violet) by resisting a decolorization process. Gram staining involves four steps. First cells are stained with crystal violet, followed by the addition of a setting agent for the stain (iodine). Then alcohol is applied, which selectively removes the stain from only the Gram negative cells. Finally, a secondary stain, safranin, is added, which counterstains the decolorized cells pink. Although Gram didn’t know it at the time, the main difference between these two types of bacterial cells is their cell walls. Gram negative cell walls have an outer membrane (also called the envelope) that dissolves during the alcohol wash. This permits the crystal violet dye to escape. Only the decolorized cells take up the pink dye safranin, which explains the difference in color between the two types of cells. At the conclusion of the Gram stain procedure, Gram positive cells appear purple, and Gram negative cells appear pink.
When you interpret a Gram stained smear, you should also describe the morphology (shape) of the cells, and their arrangement. In Figure 5, there are two distinct types of bacteria, distinguishable by Gram stain reaction, and also by their shape and arrangement. Below, describe these characteristics for both bacteria:
|
Gram positive bacterium: |
Gram negative bacterium: |
|
|
Morphology |
||
|
Arrangement |
Acid Fast Stain
Some bacteria produce the waxy substance mycolic acid when they construct their cell walls. Mycolic acid acts as a barrier, protecting the cells from dehydrating, as well as from phagocytosis by immune system cells in a host. This waxy barrier also prevents stains from penetrating the cell, which is why the Gram stain does not work with mycobacteria such as Mycobacterium, which are pathogens of humans and animals. For these bacteria, the acid–faststaining technique is used.
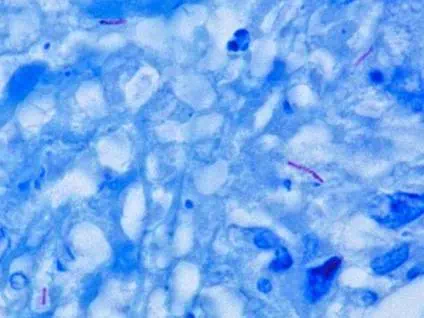
Figure 6. Acid-fast bacilli in sputum
To perform the acid-fast stain, a heat-fixed smear is flooded with the primary stain carbol fuchsin, while the slide is heated over a steaming water bath. The heat “melts” the waxy cell wall and permits the absorption of the dye by the cells. Then the slide is allowed to cool and a solution of acid and alcohol is added as a decolorizer. Cells that are “acid-fast” because of the mycolic acid in their cell wall resist decolorization and retain the primary stain. All other cell types will be decolorized. Methylene blue is then used as a counterstain. In the end, acid-fast bacteria (AFB) will be stained a bright pink color, and all other cell types will appear blue.
Staining Methods to Highlight Specific Cell Structures
Capsule: The polysaccharide goo that surrounds some species of bacteria and a few types of eukaryotic microbes is best visualized when the cells are negative stained. In this method, the bacteria are first mixed with the stain, and then a drop of the mixture is spread across the surface of a slide in the thin film. With this method, capsules appear as a clear layer around the bacterial cells, with the background stained dark.
Metachromatic granules or other intracytoplasmic bodies: Some bacteria may contain storage bodies that can be stained. One example is the Gram positive bacilli Corynebacterium, which stores phosphate in structures called “volutin” or metachromatic granules that are housed within the cell membrane. Various staining methods are used to visualize intracytoplasmic bodies in bacteria, which often provide an identification clue when observed in cells.
Endospore Stain
Endospores are dormant forms of living bacteria and should not be confused with reproductive spores produced by fungi. These structures are produced by a few genera of Gram-positive bacteria, almost all bacilli, in response to adverse environmental conditions. Two common bacteria that produce endospores are Bacillus or Clostridum. Both live primarily in soil and as symbionts of plants and animals, and produce endospores to survive in an environment that change rapidly and often. The process of endosporulation (the formation of endospores) involves several stages. After the bacterial cell replicates its DNA, layers of peptidoglycan and protein are produced to surround the genetic material. Once fully formed, the endospore is released from the cell and may sit dormant for days, weeks, or years. When more favorable environmental conditions prevail, endospores germinate and return to active duty as vegetative cells.
Mature endospores are highly resistant to environmental conditions such as heat and chemicals and this permits survival of the bacterial species for very long periods. Endospores formed millions of years ago have been successfully brought back to life, simply by providing them with water and food. Because the endospore coat is highly resistant to staining, a special method was developed to make them easier to see with a brightfield microscope. This method, called the endosporestain, uses either heat or long exposure time to entice the endospores to take up the primary stain, usually a water soluble dye such as malachite green since endospores are permeable to water. Following a decolorization step which removes the dye from the vegetative cells in the smear, the counterstain safranin is applied to provide color and contrast. When stained by this method, the endospores are green, and the vegetative cells stain pink, as shown in Figure 7.
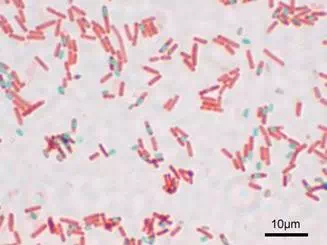
Figure 7. Bacterial cells with endospores, stained with the endospore stain.
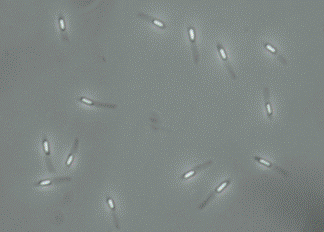
Figure 8. Bacilli with endospores viewed by phase-contrast microscopy.
Although endospores themselves are resistant to the Gram stain technique, bacterial cells captured in the process of creating these structures can be stained. In this case, the endospores are seen as clear oval or spherical areas within the stained cell. Endospores can also be directly observed in cells by using phase contrast microscopy, as shown in Figure 8.
Method
Because many differential staining methods require several steps and take a long time to complete, we will not be performing all of the differential staining methods discussed above.
Pre-stained slides will be used to visualize bacterial capsules, metachromatic granules, and acid-fast bacilli. Obtain one slide of each of the three bacteria listed in the table below. As you view these slides, make note of the “highlighted” structures. Your environmental isolate may have one or more of these cellular features, and learning to recognize them will aid in identification. These should all be viewed using the oil immersion objective lens.
|
Bacterium |
Stain |
Description or sketch of cells with the specified feature |
|
Flavobacterium capsulatum |
Capsule stain |
|
|
Corynebacterium diphtheriae |
Methylene blue(metachromatic granules) |
|
|
Mycobacterium tuberculosis |
Acid fast stain |
Gram Stain
All staining procedures should be done over a sink. The Gram stain procedure will be demonstrated, and an overview is provided in Table 1.
|
Table 1. Gram stain procedural steps. |
||
|
Step |
Procedure |
Outcome |
|
Primary stain(crystal violet) |
Add several drops of crystal violet to the smear and allow it to sit for 1 minute. Rinse the slide with water. |
Both Gram-positive and Gram-negative cells will be stained purple by the crystal violet dye. |
|
Mordant (iodine) |
Add several drops of iodine to the smear and allow it to sit for 1 minute. Rinse the slide with water. |
Iodine “sets” the crystal violet, so both types of bacteria will remain purple. |
|
Decolorization (ethanol) |
Add drops of ethanol one at atime until the runoff is clear. Rinse the slide with water. |
Gram-positive cells resist decolorization and remain purple. The dye is released from Gram-negative cells. |
|
Counterstain(safranin) |
Add several drops of safranin to the smear and allow it to sit for one minute. Rinse the slide with water and blot dry. |
Gram-negative cells will be stained pink by the safranin. This dye has no effect on Gram-positive cells, which remain purple. |
A volunteer from your lab bench should obtain cultures of the bacteria you will be using in this lab, as directed by your instructor. One of the cultures will be a Gram positive bacterium, and the other will be Gram negative. Below, write the names of the bacteria you will be using, along with the BSL for each culture:
__________________________________________________________________________________
Obtain two glass slides, and prepare a smear of each of the two bacterial cultures, one per slide, as demonstrated. Allow to COMPLETELY air dry and heat fix. Stain both smears using the Gram stain method. Observe the slides with a light microscope at 1,000X and record your observations in the table below.
|
Name of culture |
Gram stain reaction |
Cellular morphology |
Arrangement |
Gram Stain “Final Exam”: prepare a smear that contains a mixture of the Gram-positive AND Gram-negative bacteria by adding a small amount of each bacterium to a single drop of water on a slide. Heat fix the smear and Gram stain it. You should be able to determine the Gram stain reaction, cellular morphology and arrangement of BOTH bacteria in this mixed smear. Your instructor may ask to see this slide and offer constructive commentary.
Endospore Stain
Only a few genera of bacteria produce endospores and nearly all of them are Gram-positive bacilli. Most notable are Bacillus and Clostridium species, which naturally live in soil and are common contaminants on surfaces. The growth of Clostridium spp. is typically limited to anaerobic environments; Bacillus spp. may grow aerobically and anaerobically. Endospore-forming bacteria are distinct from other groups of Gram positive bacilli and distinguishable by their endospores.
An overview of the endospore stain procedure is provided in Table 2.
|
Table 2. Endospore stain procedural steps. |
||
|
Step |
Procedure |
Outcome |
|
Primary stain(malachite green) |
Add several drops of malachite green to the smear and allow it to sit for 10 minutes. If the stain starts to dry out, add additional drops. |
Vegetative cells will immediately take up the primary stain. Endospores are resistant to staining but eventually take up the dye. |
|
Decolorization(water) |
Rinse the slide under a gentle stream of water for 10-15 seconds. |
Once the endospores are stained, they remain green. A thorough rinse with water will decolorize the vegetative cells. |
|
Counterstain(safranin) |
Add several drops of safranin to the smear and allow it to sit for 1 minute. Rinse the slide and blot dry. |
Decolorized vegetative cells take up the counterstain and appear pink; endospores are light green. |
After staining, endospores typically appear as light green oval or spherical structures, which may be seen either within or outside of the vegetative cells, which appear pink.
The shape and location of the endospores inside the bacterial cells, along with whether the sporangium is either distending (D) or not distending (ND) the sides of the cell, are important characteristics that aid in differentiating among species (see Figure 9).
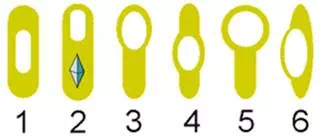
Figure 9
- Oval, central, not distended (ND)
- Oval, terminal, ND (and parasporal crystal)
- Oval, terminal, distended (D)
- Oval, central, D
- Spherical, terminal, D
- Oval, lateral, D
Endospores are quite resistant to most staining procedures; however, in a routinely stained smear, they may be visible as “outlines” with clear space within. If you observe “outlines” or what appear to be “ghosts” of cells in a Gram stained smear of a Gram-positive bacilli, then the endospore stain should also be performed to confirm the presence or absence of endospores.
A volunteer from your lab bench should obtain bacterial cultures for endospore staining, as directed by your instructor. Note that these will all be species of Bacillus. Prepare smears and stain each using the endospore staining technique. Observe the slides and note the shape and location of the endospore and the appearance of the sporangium (swollen or not swollen) in the table below:
|
Name of culture |
Endospore Shape |
Location |
Sporangium |
In addition, choose ONE of the cultures from above and Gram stain it. Record your results below in the spaces provided:
Name of Gram stained culture: __________________________________________________
Gram stain reaction and cellular morphology: ______________________________________
Are endospores visible in the Gram stained smear? _________________ If you see endospores, describe how they appear in the Gram stained preparation, and how this is similar to and different from what you see in the endospore stained preparation.