Medical imaging AI: the bubble will break in 2-3 years

The buzz around artificial intelligence (AI) in medical imaging has turned into a boom, which is turning into a bubble. When the bubble breaks, start-ups better have a solid sales strategy, according to leading Spanish researcher in quantification.
Deepening gap between funding and profit
Funding in start-ups who develop AI for medical imaging is increasingly moving away from actual value and companies should be ready in terms of self-sustainability and sales for when the tide changes, according to Angel Alberich-Bayarri, scientific-technical director of the Biomedical Imaging Research Group (GIBI230) and CEO of QUIBIM, a spin-off company of La Fe Polytechnics University Hospital in Valencia, Spain.
“The valuation of companies has increased much over their actual profits. Funds and investors that are novel to the radiology business are entering the game. There is a huge gap between how much investment a company receives and the money it actually makes,” he said.
A recent report illustrates the magnitude of the speculation around AI in medical imaging. While about 90% of the listed companies registered less than 500K in sales in 2017, many had received over $30m in funding, three over $50m and one over $70m, according to market data provider Signify Research (1). High investment means that there is a risk for the original researchers and founders to lose control and ownership of the company. Companies who did not develop a solid sales strategy or have not been acquired are also at risk when the spell fades out, Alberich-Bayarri warned.
“AI will change how we interact with everything, including radiology. Before the market auto-regulates, there will be a moment when investors understand the real value of a product and investment goes down. That’s probably going to happen in the next two to three years. To quote Warren Buffet: Only when the tide goes out, do you discover who’s been swimming naked. When that happens, I’d rather have my swimming suit on,” Alberich-Bayarri said.
Quantification tools to expedite workflows
To make sure he does, Alberich-Bayarri is developing machine-learning tools for imaging data quantification, to accelerate image reconstruction, segmentation, detection and data mining. “Advances in machine learning that help quantify imaging data can tremendously advance radiology,” he said. For image reconstruction, AI could help significantly reduce MRI acquisition times, by using raw data generated by the modality. With algorithms that process data using deep learning for under sampled MRI reconstruction, QUIBIM managed to identify all regions and tissues, and their potential variability.
Integrating all the data to do radiomics, i.e. extract and mine all the available, and even yet unknown, information on shape, texture, volume, diffusion, etc., is crucial to find new biomarkers that can help answer unsolved clinical questions. Segmentation will benefit greatly from AI. “Very soon every processing pipeline of medical imaging will have to implement segmentation, because it will help save large amounts of time,” Alberich-Bayarri said.
The U-net convolutional neural network, which was developed at Freiburg University, Germany, has considerably improved the task by drastically reducing the number of images needed for training and yielding more precision. Deep supervision to generate output segmentation masks combining multi-layer and multiresolution information is also helpful to perform segmentation.
Using these techniques, Alberich-Bayarri has managed to segment the liver on MRI, and can notably separate proximal vessels and characterize fat, iron and even search for lesions. His researchers also label images in all planes, as errors may occur when using only one network that has been trained with transversal, sagittal or coronal images. “When we combine all the information and generate a tissue probability map, liver segmentation is almost perfect,” he said.
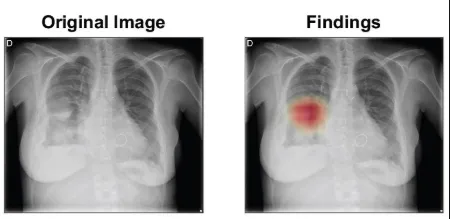
For detection, once the structure and organs are visualized, Alberich-Bayarri recommends using both supervised and non-supervised AI clustering to extract further information from an imaging scan, depending on the application. Non-supervised AI clustering can help extract and mine quantitative information that is not yet visible on imaging.
For doubtful cases, QUIBIM has developed an abnormality indicator that can be included in the radiologists’ worklist, to help them prioritise reading tasks. The abnormality score has been trained to give more weight to life-threatening findings, such as mass or big opacities.