Biopotential Measurements
Biopotential measurements are made using different kinds of specialized electrodes. The function of these recording electrodes is to couple the ionic potentials generated inside the body to an electronic instrument. Biopotential electrodes are classified either as non-invasive (skin surface) or invasive (e.g., microelectrodes or wire electrodes). Biopotential measurements must be carried out using high-quality electrodes to minimize motion artefacts and ensure that the measured signal is accurate, stable, and undistorted. Body fluids are very corrosive to metals, so not all metals are acceptable for biopotential sensing. Furthermore, some materials are toxic to living tissues. For implantable applications, we typically use relatively strong metal electrodes made, for example, from stainless steel or noble materials such as gold, or from various alloys such as platinum-tungsten, platinum iridium, titanium-nitride, or iridium-oxide. These electrodes do not react chemically with tissue electrolytes and therefore minimize tissue toxicity. Unfortunately, they give rise to large interface impedances and unstable potentials. External monitoring electrodes can use nonnoble materials such as silver with lesser concerns of biocompatibility, but they must address the large skin interface impedance and the unstable biopotential. Other considerations in the design and selection of biopotential electrodes are cost, shelf life, and mechanical characteristics.
The Electrolyte/Metal Electrode Interface
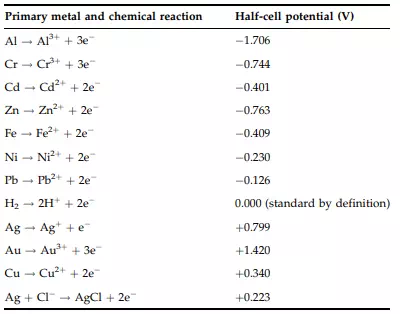
When a metal is placed in an electrolyte (i.e., an ionizable) solution, a charge distribution is created next to the metal/electrolyte interface, as illustrated in Figure 10.5. This localized charge distribution causes an electric potential, called a half-cell potential, to be developed across the interface between the metal and the electrolyte solution. The half-cell potentials of several important metals are listed in Table 10.2. Note that the hydrogen electrode is considered to be the standard electrode against which the half-cell potentials of other metal electrodes are measured.
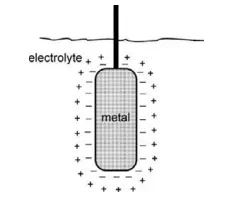
FIGURE 10.5 Distribution of charges at a metal/electrolyte interface.
TABLE 10.2 Half-Cell Potentials of Important Metals
Typically, biopotential measurements are made by utilizing two similar electrodes composed of the same metal. Therefore, the two half-cell potentials for these electrodes would be equal in magnitude. For example, two similar biopotential electrodes can be taped to the chest near the heart to measure the electrical potentials generated by the heart (electrocardiogram, or ECG). Ideally, assuming that the skin-to-electrode interfaces are electrically identical, the differential amplifier attached to these two electrodes would amplify the biopotential (ECG) signal, but the half-cell potentials would be cancelled out. In practice, however, disparity in electrode material or skin contact resistance could cause a significant DC offset voltage that would cause a current to flow through the two electrodes. This current will produce a voltage drop across the body. The offset voltage will appear superimposed at the output of the amplifier and may cause instability or base line drift in the recorded biopotential.
ECG Electrodes
Examples of different types of non-invasive biopotential electrodes used primarily for ECG recording are shown in Figure 10.6. A typical flexible biopotential electrode for ECG recording is composed of certain types of polymers or elastomers that are made electrically conductive by the addition of a fine carbon or metal powder. These electrodes (Figure 10.6a) are available with prepasted AgCl gel for quick and easy application to the skin using a double-sided peel-off adhesive tape.
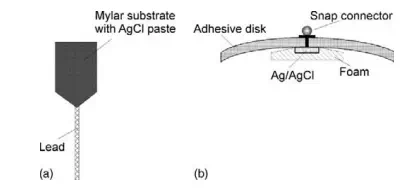
FIGURE 10.6 Biopotential skin surface ECG electrodes: (a) flexible Mylar electrode and (b) disposable snap type Ag/AgCl electrode.
The most common type of biopotential electrode is the silver/silver chloride electrode (Ag/AgCl), which is formed by electrochemically depositing a very thin layer of silver chloride onto a silver electrode (Figure 10.6b). These electrodes are recessed from the surface of the skin and imbedded in foam that has been soaked with an electrolyte paste to provide good electrical contact with the skin. The electrolyte-saturated foam is also known to reduce motion artefacts that could be produced, for example, during stress testing when the layer of the skin moves relative to the surface of the Ag/AgCl electrode. This motion artefact could cause large interference in the recorded biopotential and, in extreme cases, could severely degrade the measurement.