What are Van der Waals Forces?
Van der Waals forces are weak intermolecular forces that are dependent on the distance between atoms or molecules. These forces arise from the interactions between uncharged atoms/molecules.
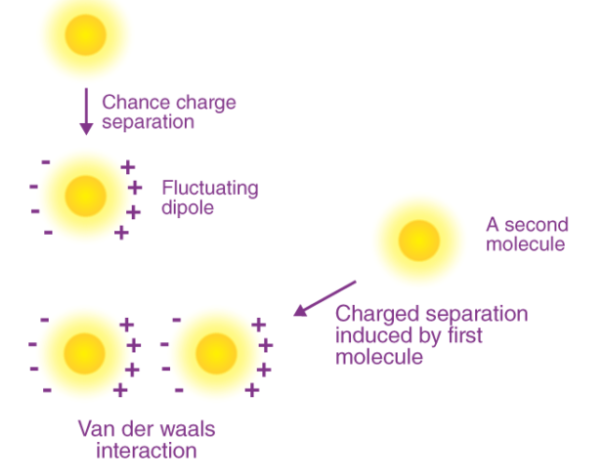
For example, Van der Waals forces can arise from the fluctuation in the polarizations of two particles that are close to each other.
In the group of forces that fall under the category of ‘weak chemical forces’, Van der Waals forces are the weakest. They are known to rapidly vanish when the distance between the interacting molecules increases. The strengths of Van der Waals forces typically range from 0.4 kJ.mol-1 to 4 kJ.mol-1.
When the electron density around the nucleus of an atom undergoes a transient shift, it is common for Van der Waals forces arising. For example, when the electron density increases in one side of the nucleus, the resulting transient charge may attract or repel a neighbouring atom. The nature of these forces is dependent on the distance between the atoms:

An illustration detailing the induced formation of a dipole in an atom/molecule due to a fluctuating dipole in another atom/molecule is provided above. The adsorption of gaseous molecules to the surface of an adsorbent and the cohesion of condensed phases can be accounted for by Van der Waals forces.
Characteristics of Van der Waals Forces