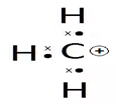
Carbocation
A carbocation is a molecule in which a carbon atom has a positive charge and three bonds. We can basically say that they are carbon cations. Formerly, it was known as carbonium ion. Carbocation today is defined as any even-electron cation that possesses a significant positive charge on the carbon atom.
Talking about some general characteristics, the carbon cations are very reactive and unstable due to an incomplete octet. In simple words, carbocations do not have eight electrons, therefore they do not satisfy the octet rule.

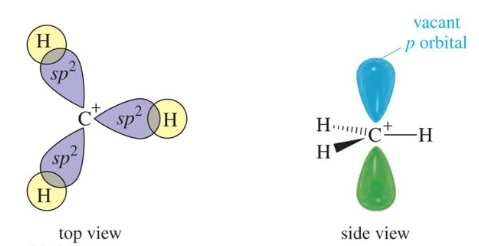
In carbocation, the hybridization of carbon will be sp2 and its shape is trigonal planar. There is also a vacant p orbital which indicates its electron-deficient nature. The carbon has 6 electrons in its valence shell. Due to this, it is an electron-deficient species, also known as an electrophile.
A carbocation is generally observed in an SN1 reaction, elimination reaction, etc.
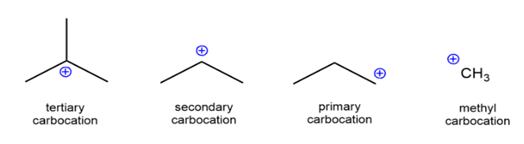
Classification of Carbocation
The different carbocations are named on the basis of the number of carbon groups bonded to the carbon. The carbocation can be termed as methyl, primary, secondary or tertiary on the basis of how many carbon atoms are attached to it:
Interestingly, in addition to these types, there is another type of carbocation which is known as pyramidal carbocation. In this type, the ions consist of a single carbon atom that usually tends to hover over a four- or five-sided polygon which can be depicted as a pyramid. The 4 sided pyramidal ion will consist of +1 charge while the five-sided pyramid will have +2 charge.
Formation of the Carbocation
The carbocations can be formed by either of the following two fundamental steps:
Cleavage of Bond of Carbon
Whenever there is cleavage of the bond of carbon and atoms attached to it, the leaving group takes away the shared electrons. Thus leaving the carbon atom as electron deficient. As a result, a positive charge is developed forming a carbocation. The more tendency of cleavage of bond or formation of a more stable carbocation the lower is the activation energy.
In many organic reactions such as the SN1 and E1 reactions carbocation is formed as a reaction intermediate.
Electrophilic Addition
In electrophilic addition, an electrophile attacks on unsaturated point(double or triple bond), this results in the breaking of the pi bond which results in the formation of a carbocation. The more stable is the carbocations the lower is the activation energy and faster addition. Electrophilic addition to a pi bond is illustrated by the reaction of HBr (an electrophile) with propene (CH3CH = CH2).
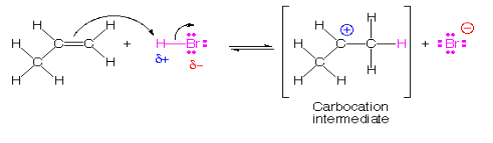
It can be noted that the formation of the secondary carbocation is favoured over the primary carbocation because secondary carbocation is more stabilized due to resonance. This is also in accordance with Markovnikov’s Rule. Such electrophilic addition reactions are generally seen in alkenes, alkynes and benzene rings.
As we know that the carbocations are very reactive due to their electron deficiency, vacant orbital and incomplete octet. Therefore, its stability depends on the octet completion and reducing the electron deficiency.
The stability of a carbocation can be achieved by the following processes:
(a) Addition of a nucleophile.
(b) Formation of a pi bond.
(c) Rearrangement.
Addition of a Nucleophile
A carbocation is electron-deficient and with an incomplete octet and a positive charge on it. The positive charge is stabilized by the addition of a nucleophile thus the formation of a new covalent bond takes place. This stabilizes the carbocation. This is a very common process of stabilization of carbocation because the carbocation is very reactive so even weak nucleophile gets attached to the carbocation.
Formation of a Pi Bond
The carbocation can receive electrons form nearby hydrogen to remove its positive charge and to complete its octet. Thus a new pi bond can be formed. The hydrogen atom is generally must be removed by any base. Due to the high reactivity of the carbocations even a weak base such as water or iodide ion are able to facilitate the deprotonation. Whenever such deprotonation occurs two types of products are formed. The more stable compound is the major product.
Rearrangement
The bonding electrons of a carbocation can be shifted between adjacent atoms so that more stable carbocation can be formed. For instance, rearrangement will be highly favoured if there is a conversion of a secondary carbocation can be formed from a primary carbocation The reason is simple because the carbocation is more stabilized in secondary carbocation than in a primary carbocation.
The different types of carbocation rearrangement are:
Hydride Shifting
Here hydrogen is shifted from 1st carbon to 2nd carbon. So the carbocation has changed from primary to the secondary carbocation. Thus forming a more stable structure.

Methyl shifting
Here methyl group shifts to the primary carbon to form a more stable structure. The carbocation is secondary carbocation, so more stable than primary carbocation.
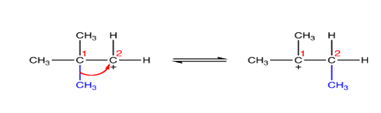
Phenyl shifting
The entire phenyl group can also be shifted to obtain a more stable secondary or tertiary carbocation than a primary carbocation. This is also interesting to know that phenyl shift is more favoured than a methyl shift.
Carbocation Stability
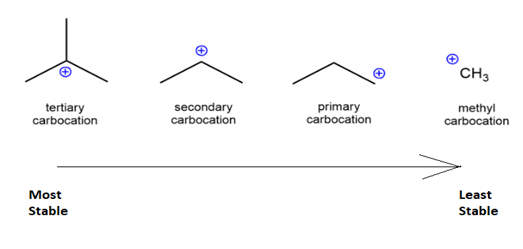
The stability order of carbocation is as follows:
The stability of carbocations depends on the following factors:
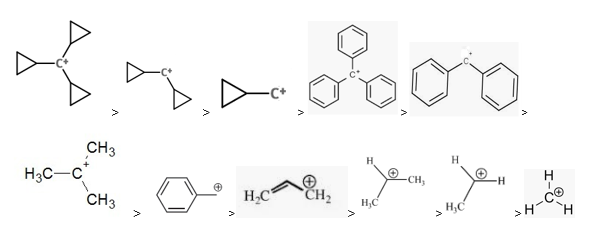
1. Resonance: Stability of carbocations increases with the increasing number of resonance. More the number of resonating structures more is the stability of the carbocation. The reason for this is the delocalization of the positive charge. The electron deficiency is decreased due to the delocalization and thus it increases the stability.
When compared to substitution, the resonance effect proves to be a more dominating factor than substitution. Therefore, structures with resonance are far better stabilised than others. Cyclopropane carbocation is exceptionally very stable due to dancing resonance. Thus tricyclo propane carbocation is the most stable carbocation.
2. Hyperconjugation and inductive effect: Increasing substitution, increases the hyperconjugation and thus it increases stability. More the hyperconjugation more is the stability.
R3C+ (3o ; most stable) > R2CH+ (2o ) > RCH2+ (1o) CH3+ (methyl; least stable)
The Carbocation stability depends on the number of carbon groups attached to the carbon carrying the positive charge.
3. Electronegativity: Electronegativity indicates the capacity of an atom to attract electrons. The more is the electronegativity, the more is the attraction of the electrons towards the atom. Therefore the electronegativity of the carbon with the positive directly affects the stability of the carbocation. So as the electronegativity of the carbon atom increases the stability of the carbocation decreases. sp > sp2 > sp3 ( sp has maximum s character; so maximum electronegativity, sp3 has minimum s character; so minimum electronegativity).
The hybridisation of the carbon with the positive charge in the vinylic carbocation is sp whose electronegativity is more than the sp2 hybridized carbon of the alkyl carbocation. Due to this reason, the stability of a primary vinylic carbocation is less than a primary alkyl carbocation.