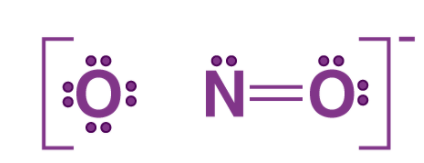
What is Ionic Bond?
The bond formed as a result of strong electrostatic forces of attraction between a positively and negatively charged species is called an electrovalent or ionic bond. The positively and negatively charged ions are aggregated in an ordered arrangement called the crystal lattice which is stabilized by the energy called the Lattice enthalpy.
Conditions for the formation of an Ionic Bond
Generally, the ionic bond is formed between a metal cation and non-metal anion.
The following steps are adopted for writing the Lewis dot structures or Lewis structures:
Step 1: Calculate the number of electrons required for drawing the structure by adding the valence electrons of the combining atoms. For Example, in methane, CH4 molecule, there are 8 valence electrons (in which 4 belongs to carbon while other 4 to H atoms).
Step 2: Each negative charge i.e. for anions, we add an electron to the valence electrons and for each positive charge i.e. for cations we subtract one electron from the valence electrons.
Step 3: Using the chemical symbols of the combining atoms and constructing a skeletal structure of the compound, divide the total number of electrons as bonding shared pairs between the atoms in proportion to the total bonds.
Step 4: The central position in the molecule is occupied by the least electronegative atom. Hydrogen and fluorine generally occupy the terminal positions.
Step 5: After distributing the shared pairs of electrons for single bonds, the remaining electron pairs are used for multiple bonds or they constitute lone pairs.
The basic requirement is that each bonded atom gets an octet of electrons.
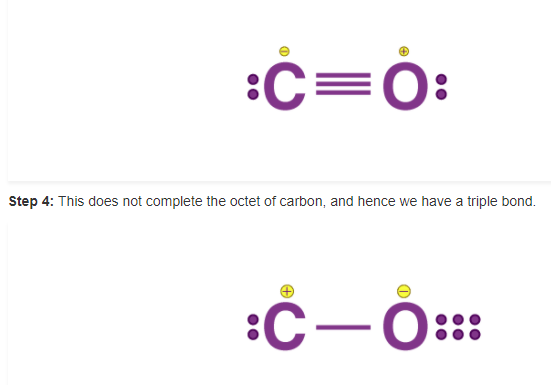
Example 1: Lewis formula for carbon monoxide, CO
Step 1: Counting the total number of valence electrons of carbon and oxygen atoms: C (2s22p2) + O (2s22p4) 4 + 6 = 10 that is, 4(C) + 6(O) = 10
Step 2: The skeletal structure of carbon monoxide is written as CO
Step 3: Drawing a single bond between C and O and completing octet on O, the remaining two electrons are lone pair on C.

Example 2: Lewis Structure of nitrite, NO2–
Step 1: Counting the total number of valence electrons of one nitrogen atom, two oxygen atoms and the additional one negative charge (equal to one electron). Total Number of valence electrons is: N (2s22p3) + 2O (2s22p4) + 1 (negative charge) => 5+ 2(6) +1=18e–
Step 2: The skeletal structure of nitrite ion is written as O-N-O
Step 3: Drawing a single bond between nitrogen and each oxygen atom: O – N – O
Step 4: Complete the octets of atoms.

This structure does not complete octet on N if the remaining two electrons constitute of a lone pair on it. Therefore, we have a double bond between one N and one of the two O atoms. The Lewis structure is