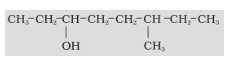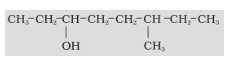
A functional group is an individual atom or a compound formed uniquely. A functional group is the most reactive site in an organic molecule. Compounds with a similar functional group have the similar type of reactions.
For example, CH3OH and CH3CH2OH have the similar type of reactions as they have the same functional group OH. These liberate hydrogen on reaction with sodium metal.
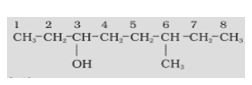
In an organic compound, firstly, the functional group is identified which gives us the appropriate suffix. Then the longest carbon chain having the functional group is chosen in such a manner that the functional group gets the lowest number in the chain.
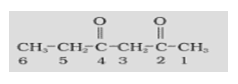
In case of multiple functional groups in a compound, one of the functional groups is chosen on the basis of the priority list and is referred to as the principal functional group. The compound is named on the basis of that functional group. Remaining functional groups are known as the substituents and are named using the appropriate prefixes.
The priority list of the functional group can be given as:
-COOH > -SO3H > -COOR (R= alkyl group) > -COCl > -CONH2 > -CN > -HC=O > >C=O > -OH > -NH2 > >C=C< > -CC-
Functional groups like -R, C6H5-, halogens (F, Cl, Br, I), -NO2, alkoxy (-OR) etc., are always used as prefix substituents.
If more than one same functional group is present in the compound, then they are indicated as di, tri, … etc. before the suffix and full name of parent alkane is written.