What are Organic Compounds?
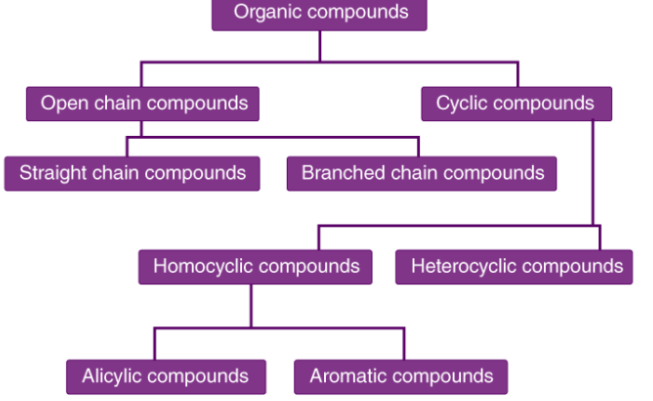
The compounds in solid, liquid or gaseous state which contain carbon in its molecule are known as organic compounds. There are a large number of organic compounds and therefore a proper systematic classification was required. Organic compounds can be broadly classified as acyclic (open chain) or cyclic (closed chain). Moving on to their classification in detail:

Organic compounds are seen in a number of formats, including Lewis structures, space filled models and structural formulas. It is not uncommon to view the hydrogens as lines or to leave them all together in a structural formula of an organic molecule. They are understood to be present in order to complete the 4-bonds provided by the carbon atoms.
Organic compounds have been detected by mass spectra. The results obtained indicate that all extractants were able to perform direct analysis after extraction without any clean-up phase. Nevertheless, the form of organic compounds extracted depended on the solvent used, suggesting their distinct ability to solubilize various biosolid organic compounds.
Organic chemistry was once thought to be confined to the study of substances produced as part of the natural processes of living organisms, but as Friedrich Wohler discovered in the early 1800s, organic compounds can be synthesized from minerals and other non-organic materials in the laboratory. Indeed, modern chemistry and materials sciences have concentrated on the remarkable properties of carbon atoms for the production of synthetic chemicals, pesticides and a host of other things. Organic compounds contain carbon, almost always bonded to another carbon and/or hydrogen.
Sometimes, other elements, such as phosphorus, nitrogen and oxygen, are also bound to carbons. There are a few carbon compounds that are not considered organic molecules. Those involve carbon dioxide, carbon monoxide, cyanates, cyanides and other carbon-containing ion compounds. Other elements, such as phosphorus, nitrogen and oxygen, are also sometimes bound to carbon. There are a few carbon compounds which are not considered organic molecules. Those involve carbon dioxide, carbon monoxide, cyanates, cyanides and other carbon-containing ion compounds.
Alcohols include chemicals such as ethanol and isopropanol. They are used as antiseptics and ethanol is a staple of the beverage industry. Finally, carboxylic acids include a wide range of chemicals, including pharmaceuticals. Aspirin, one of the oldest commercial medicines, contains carboxylic acid.
While there are millions of organic compounds, there is a fairly simple classification scheme for these compounds and a method for naming even the most complex organic compounds. This unit will focus on helping you to identify the classification of organic compounds and to name only some of the most common of these compounds.