Types of Hybridization
Based on the types of orbitals involved in mixing, the hybridization can be classified as sp3, sp2, sp, sp3d, sp3d2, sp3d3. Let us now discuss the various types of hybridization, along with their examples.
sp Hybridization
sp hybridization is observed when one s and one p orbital in the same main shell of an atom mix to form two new equivalent orbitals. The new orbitals formed are called sp hybridized orbitals. It forms linear molecules with an angle of 180°
- This type of hybridization involves the mixing of one ‘s’ orbital and one ‘p’ orbital of equal energy to give a new hybrid orbital known as a sp hybridized orbital.
- sp hybridization is also called diagonal hybridization.
- Each sp hybridized orbital has an equal amount of s and p character, i.e., 50% s and p character.
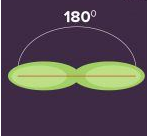
Examples of sp Hybridization:
- All compounds of beryllium like BeF2, BeH2, BeCl2
- All compounds of carbon-containing triple Bond like C2H2.
sp2 Hybridization
sp2 hybridization is observed when one s and two p orbitals of the same shell of an atom mix to form 3 equivalent orbital. The new orbitals formed are called sp2 hybrid orbitals.
- hy2 bridization is also called trigonal hybridization.
- It involves mixing of one ‘s’ orbital and two ‘p’ orbital’s of equal energy to give a new hybrid orbital known as sp2.
- A mixture of s and p orbital formed in trigonal symmetry and is maintained at 1200.
- All the three hybrid orbitals remain in one plane and make an angle of 120° with one another. Each of the hybrid orbitals formed has 33.33% s character and 66.66% ‘p’ character.
- The molecules in which the central atom is linked to 3 atoms and is sp2 hybridized have a triangular planar shape.
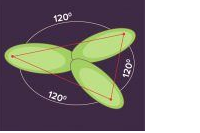
Examples of sp2 Hybridization
- All the compounds of Boron i.e. BF3, BH3
- All the compounds of carbon containing a carbon-carbon double bond, Ethylene (C2H4)
sp3 Hybridization
When one ‘s’ orbital and 3 ‘p’ orbitals belonging to the same shell of an atom mix together to form four new equivalent orbital, the type of hybridization is called a tetrahedral hybridization or sp3. The new orbitals formed are called sp3 hybrid orbitals.
- These are directed towards the four corners of a regular tetrahedron and make an angle of 109°28’ with one another.
- The angle between the sp3 hybrid orbitals is 109.280
- Each sp3 hybrid orbital has 25% s character and 75% p character.
- Example of sp3 hybridization: ethane (C2H6), methane.
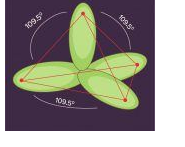
sp3d Hybridization
sp3d hybridization involves the mixing of 3p orbitals and 1d orbital to form 5 sp3d hybridized orbitals of equal energy. They have trigonal bipyramidal geometry.
- The mixture of s, p and d orbital forms trigonal bipyramidal symmetry.
- Three hybrid orbitals lie in the horizontal plane inclined at an angle of 120° to each other known as the equatorial orbitals.
- The remaining two orbitals lie in the vertical plane at 90 degrees plane of the equatorial orbitals known as axial orbitals.
- Example: Hybridization in Phosphorus pentachloride (PCl5)
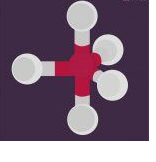
sp3d2 Hybridization
- Sp3d2 hybridization has 1s, 3p and 2d orbitals, that undergo intermixing to form 6 identical sp3d2 hybrid orbitals.
- These 6 orbitals are directed towards the corners of an octahedron.
- They are inclined at an angle of 90 degrees to one another.
Key Features of Hybridization
- Atomic orbitals with equal energies undergo hybridization.
- The number of hybrid orbitals formed is equal to the number of atomic orbitals mixing.
- It is not necessary that all the half-filled orbitals must participate in hybridization. Even completely filled orbitals with slightly different energies can also participate.
- Hybridization happens only during the bond formation and not in an isolated gaseous atom.
- The shape of the molecule can be predicted if hybridization of the molecule is known.
- The bigger lobe of the hybrid orbital always has a positive sign, while the smaller lobe on the opposite side has a negative sign.


