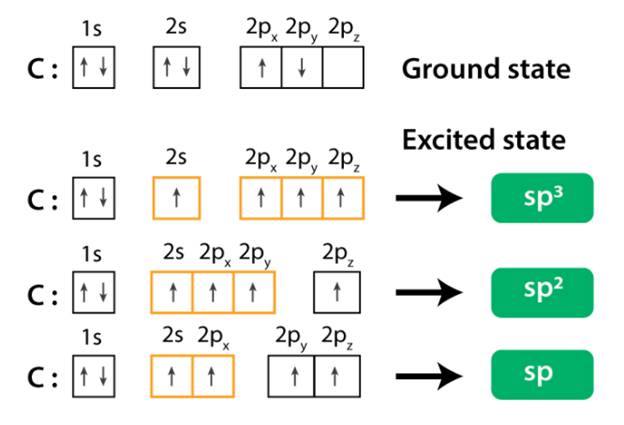
What is meant by hybridisation
Hybridization is the combining of atomic orbitals to produce new orbitals of varying energies and shapes than the initial orbitals. Hybridization occurs as atomic orbitals combine to create a new atomic orbital. The new orbital will contain the same total electron number as the old electrons. The idea of hybridization was developed because the fact that all the C-H bonds in molecules such as methane are similar was the only reason.

The object of hybridization is to see the types of bonds, whether sigma or pi bonds, that the atoms share with each other. Various properties are permitted by the various forms of bonds, such as how pi bonds do not support rotation whereas sigma bonds are rotational.