What are Molecular Orbitals?
The space in a molecule in which the probability of finding an electron is maximum can be calculated using the molecular orbital function. Molecular orbitals are basically mathematical functions that describe the wave nature of electrons in a given molecule.
These orbitals can be constructed via the combination of hybridized orbitals or atomic orbitals from each atom belonging to the specific molecule. Molecular orbitals provide a great model via the molecular orbital theory to demonstrate the bonding of molecules.
Types of Molecular Orbitals
According to the molecular orbital theory, there exist three primary types of molecular orbitals that are formed from the linear combination of atomic orbitals. These orbitals are detailed below.
Anti Bonding Molecular Orbitals
The electron density is concentrated behind the nuclei of the two bonding atoms in anti-bonding molecular orbitals. This results in the nuclei of the two atoms being pulled away from each other. These kinds of orbitals weaken the bond between two atoms.
Non-Bonding Molecular Orbitals
In the case of non-bonding molecular orbitals, due to a complete lack of symmetry in the compatibility of two bonding atomic orbitals, the molecular orbitals formed have no positive or negative interactions with each other. These types of orbitals do not affect the bond between the two atoms.
Formation of Molecular Orbitals
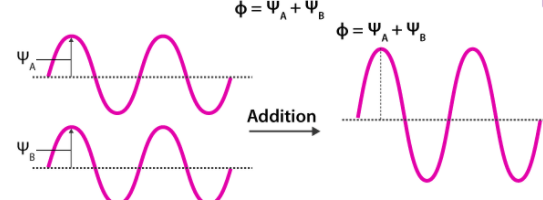
An atomic orbital is an electron wave; the waves of the two atomic orbitals may be in phase or out of phase. Suppose ΨA and ΨB represent the amplitude of the electron wave of the atomic orbitals of the two atoms A and B.
Case 1: When the two waves are in phase so that they add up and amplitude of the wave is Φ= ΨA + ΨB

Case 2: when the two waves are out of phase, the waves are subtracted from each other so that the amplitude of the new wave is Φ ´= ΨA – ΨB

The energy levels of bonding molecular orbitals are always lower than those of anti-bonding molecular orbitals. This is because the electrons in the orbital are attracted by the nuclei in the case of bonding Molecular Orbitals whereas the nuclei repel each other in the case of the anti-bonding Molecular Orbitals.