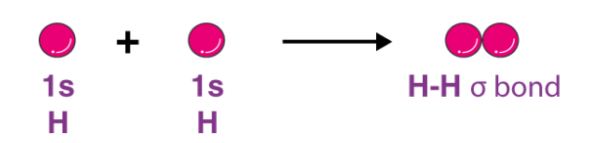
What is Valance Bond (VB) Theory?
The metal bonding is essentially covalent in origin and metallic structure involves resonance of electron-pair bonds between each atom and its neighbors.
The Lewis approach to chemical bonding failed to shed light on the formation of chemical bonds. Also, valence shell electron pair repulsion theory (or VSEPR theory) had limited applications (and also failed in predicting the geometry corresponding to complex molecules).
In order to address these issues, the valence bond theory was put forth by the German physicists Walter Heinrich Heitler and Fritz Wolfgang London. The Schrodinger wave equation was also used to explain the formation of a covalent bond between two hydrogen atoms. The chemical bonding of two hydrogen atoms as per the valence bond theory is illustrated below.
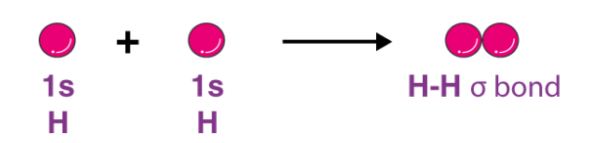
This theory focuses on the concepts of electronic configuration, atomic orbitals (and their overlapping) and the hybridization of these atomic orbitals. Chemical bonds are formed from the overlapping of atomic orbitals wherein the electrons are localized in the corresponding bond region.
The valence bond theory also goes on to explain the electronic structure of the molecules formed by this overlapping of atomic orbitals. It also emphasizes that the nucleus of one atom in a molecule is attracted to the electrons of the other atoms.
The important postulates of the valence bond theory are listed below.
The formation of sigma and pi bonds is illustrated below.
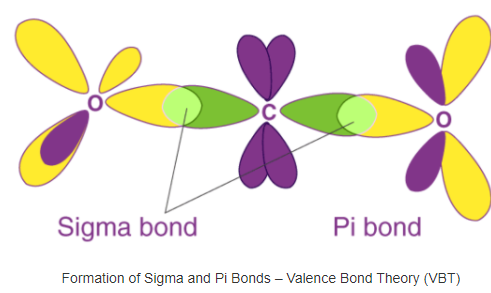
It can be noted that sigma bonds involve the head-to-head overlapping of atomic orbitals whereas pi bonds involve parallel overlapping.
Number of Orbitals and Types of Hybridization
According to VBT theory the metal atom or ion under the influence of ligands can use its (n-1)d, ns, np, or ns, np, nd orbitals for hybridization to yield a set of equivalent orbitals of definite geometry such as octahedral, tetrahedral, square planar and so on. These hybrid orbitals are allowed to overlap with ligand orbitals that can donate electron pairs for bonding.
Coordination Number | Type of Hybridisation | Distribution of Hybrid Orbitals in Space |
4 | sp3 | Tetrahedral |
4 | dsp2 | Square planar |
5 | sp3d | Trigonal bipyramidal |
6 | sp3d2 | Octahedral |
6 | d2sp3 | Octahedral |
Applications of Valence Bond Theory
Limitations of Valence Bond Theory
The shortcomings of the valence bond theory include