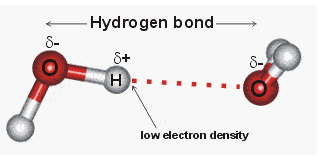
How is Hydrogen Bond Formed
Molecules of water form hydrogen bonds with each other. A hydrogen bond with a partial positive charge on the hydrogen of other molecules may form a partial negative charge on the O of one molecule. Water molecules are also attracted to ions and to other polar molecules.
Hydrogen bonding, a hydrogen atom interaction found between a pair of other atoms with a high electron affinity; such a bond is weaker than an ionic bond or covalent bond, but greater than the powers of van der Waals.

Hydrogen bonding, not a covalent bond to a hydrogen atom, is a special form of dipole-dipole attraction between molecules. It arises from the attractive force between a covalently bound hydrogen atom with a very electronegative atom such as an atom of N, O, or F and another atom that is very electronegative.