Sweet, Sweet Carbs
 Carbohydrate is a fancy way of saying "sugar." Scientists
came up with the name because the molecule have many carbon (C)
atoms bonded to hydroxide (OH-) groups. Carbohydrates can be very
small or very large molecules, but they are still considered sugars. Plants can
create long chains of these molecules for food storage or structural reasons.
Carbohydrate is a fancy way of saying "sugar." Scientists
came up with the name because the molecule have many carbon (C)
atoms bonded to hydroxide (OH-) groups. Carbohydrates can be very
small or very large molecules, but they are still considered sugars. Plants can
create long chains of these molecules for food storage or structural reasons.
What's It Used For?
A
carbohydrate is called an organic compound, because it is made up of a long
chain of carbon atoms. Sugars provide living things with energy and act as
substances used for structure. When sugars are broken down in the mitochondria, they can
power cell machinery to create the energy-rich compound called ATP (adenosine
triphosphate). Some examples of structural uses might be the shell of a crab
(chitin) or the stem of a plant (cellulose). We'll talk about them in a little
bit.
Saccharides
 Scientists also use the word saccharide to describe
sugars. If there is only one sugar molecule, it is called a monosaccharide. If
there are two, it is a disaccharide. If there are three, it is a trisaccharide.
You get the idea.
Scientists also use the word saccharide to describe
sugars. If there is only one sugar molecule, it is called a monosaccharide. If
there are two, it is a disaccharide. If there are three, it is a trisaccharide.
You get the idea.
Simple Sugars
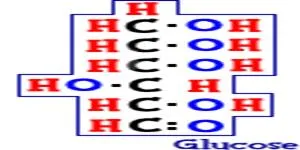
What
about the simplest of sugars? A sugar called glucose is the most important
monosaccharide on Earth. Glucose (C6H12O6)
is created by photosynthesis and
used in cellular respiration. When you think of table sugar, like the kind in
candy, it is actually a disaccharide. The sugar on your dinner table is made of
glucose and another monosaccharide called fructose (C6H12O6).
These sugars have the same numbers of atoms, but they are different
structures called isomers.
Polysaccharides
When
several carbohydrates combine, it is called a polysaccharide ("poly"
means many). Hundreds of sugars can be combined in a branched chain. These
chains are also known as starches.
You can find starches in foods such as pasta and potatoes. They are very good
sources of energy for your body.
Sugars for Structure and Support
 An important structural polysaccharide is cellulose. Cellulose is
found in plants. It is one of those carbohydrates used to support or protect an
organism. Cellulose is in wood and the cell walls of plants. You know that
shirt you're wearing? If it is made of cotton, that's cellulose, too! There can
be thousands of glucose subunits in one large molecule of cellulose. If we were
like some herbivores or insects, such as termites, we could eat cellulose for
food. Those animals don't actually digest the polysaccharides. They have small
microorganisms in their bellies that break down the molecules and release
smaller sugars.
An important structural polysaccharide is cellulose. Cellulose is
found in plants. It is one of those carbohydrates used to support or protect an
organism. Cellulose is in wood and the cell walls of plants. You know that
shirt you're wearing? If it is made of cotton, that's cellulose, too! There can
be thousands of glucose subunits in one large molecule of cellulose. If we were
like some herbivores or insects, such as termites, we could eat cellulose for
food. Those animals don't actually digest the polysaccharides. They have small
microorganisms in their bellies that break down the molecules and release
smaller sugars.
Polysaccharides are also used in the shells
(chitin) of crustaceans, such as crabs and lobsters. Chitin is similar in
some ways to the structure of cellulose, but has a far different use. The
shells are solid, protective structures that need to be molted (left behind)
when the crustacean begins to grow. It is very inflexible. On the other hand,
it is very resistant to damage. While a plant may burn, it takes very high
temperatures to hurt the shell of a crab. If you know the way crabs are cooked,
you know that the crab meat cooks on the inside of the shells when it is
boiled. There is no damage to the shells at the temperature of boiling water (H2O
at 100oC).