Introduction to Biochemistry
Biochemistry is the study of chemical processes in living organisms, including, but not limited to, living matter. Biochemistry governs all living organisms and living processes. By controlling information flow through biochemical signaling and the flow of chemical energy through metabolism, biochemical processes give rise to the incredible complexity of life.
Over the last decades of the 20th century, biochemistry become so successful at explaining living processes that now almost all areas of the life sciences from botany to medicine to genetics are engaged in biochemical research. Today, the main focus of pure biochemistry is in understanding how biological molecules give rise to the processes that occur within living cells, which in turn relates greatly to the study and understanding of whole organisms.
Biochemistry is closely related to molecular biology, the study of the molecular mechanisms by which genetic information encoded in DNA is able to result in the processes of life. Depending on the exact definition of the terms used, molecular biology can be thought of as a branch of biochemistry, or biochemistry as a tool with which to investigate and study molecular biology.
Much of biochemistry deals with the structures, functions and interactions of biological macromolecules, such as proteins, nucleic acids, carbohydrates and lipids, which provide the structure of cells and perform many of the functions associated with life. The chemistry of the cell also depends on the reactions of smaller molecules and ions. These can be inorganic, for example water and metal ions, or organic, for example the amino acids which are used to synthesize proteins. The mechanisms by which cells harness energy from their environment via chemical reactions are known as metabolism. The findings of biochemistry are applied primarily in medicine, nutrition, and agriculture. In medicine, biochemists investigate the causes and cures of disease. In nutrition, they study how to maintain health and study the effects of nutritional deficiencies. In agriculture, biochemists investigate soil and fertilizers, and try to discover ways to improve crop cultivation, crop storage and pest control. Much of biochemistry deals with the structures and functions of cellular components such as proteins, carbohydrates, lipids, nucleic acids and other biomolecules—although increasingly processes rather than individual molecules are the main focus.
Proteins
Proteins, from the Greek proteios, meaning first, are a class of organic compounds which are present in and vital to every living cell. In the form of skin, hair, callus, cartilage, muscles, tendons and ligaments, proteins hold together, protect, and provide structure to the body of a multi-celled organism. In the form of enzymes, hormones, antibodies, and globulins, they catalyze, regulate, and protect the body chemistry. In the form of hemoglobin, myoglobin and various lipoproteins, they effect the transport of oxygen and other substances within an organism.
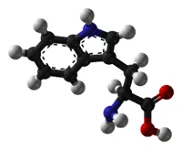 Amino Acids
Amino Acids
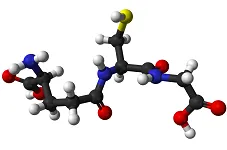 Peptides and Proteins
Peptides and Proteins
 Protein Structure
Protein Structure
 Case Studies: Proteins
Case Studies: Proteins
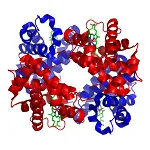
Thumbnail: Structure of human hemoglobin. The proteins α and β subunits are in red and blue, and the iron-containing heme groups in green.
Lipids
Lipids are biomolecules that are soluble in organic non-polar solvents and are hence insoluble in water. Glycerides and waxes form a sub-group, which have an ester as the major functional group and include triglycerides and phospholipids. Another diverse group of compounds which do not have any ester functional groups are also classified as lipids. Lipids without ester functional groups include: steroids, fatty acids, soaps, sphingolipids, and prostaglandins.
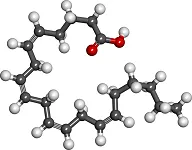 Fatty Acids
Fatty Acids
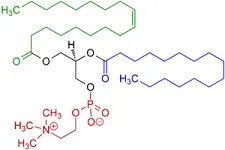 Glycerides
Glycerides
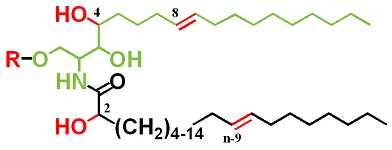 Non-glyceride Lipids
Non-glyceride Lipids
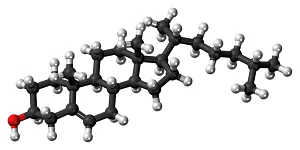 Steroids
Steroids
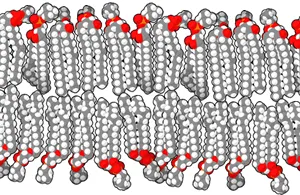 Applications of Lipids
Applications of Lipids