Extrinsic semiconductors
Semiconductors can be broadly classified into Intrinsic and Extrinsic Semiconductors. Intrinsic Semiconductors start conducting at temperatures above the room temperature, developing important electronic devices using these can pose a problem. This led to a need for improving the conductivity of intrinsic semiconductors.
After some experiments, scientists observed an increase in the conductivity of a Semiconductor when a small amount of impurity was added to it. These materials are Extrinsic Semiconductors or impurity Semiconductors. Another term for these materials is ‘Doped Semiconductor’. The impurities are dopants and the process – Doping.
An important condition to doping is that the amount of impurity added should not change the lattice structure of the Semiconductor. To achieve this the size of the dopant and Semiconductor atoms should be the same.
Crystals of Silicon and Germanium are doped using two types of dopants:
1. Pentavalent (valency 5); like Arsenic (As), Antimony (Sb), Phosphorous (P), etc.
2. Trivalent (valency 3); like Indium (In), Boron (B), Aluminium (Al), etc.
The reason behind using these dopants is to have similarly sized atoms as the pure semiconductor. Both Si and Ge belong to the fourth group in the periodic table. Hence, the choice of dopants is from the third and fifth group. This ensures that size of the atoms is not much different from the fourth group. Hence, the trivalent and pentavalent choices. These dopants give rise to two types of semiconductors:
1. n-type
2. p-type
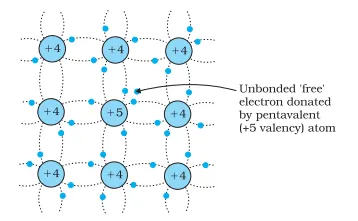
An n-type semiconductor is created when pure semiconductors, like Si and Ge, are doped with pentavalent elements.

As can be seen in the image above, when a pentavalent atom takes the place of a Si atom, four of its electrons bond with four neighbouring Si atoms. However, the fifth electron remains loosely bound to the parent atom. Hence, the ionization energy required to set this electron free is very small. Thereby, this electron can move in the lattice even at room temperature.
To give you a better perspective, the ionization energy required for silicon at room temperature is around 1.1 eV. On the other hand, by adding a pentavalent impurity, this energy drops to around 0.05 eV.
It is important to remember that the number of electrons made available by the dopant atoms is independent of the ambient temperature and primarily depends on the doping level. Also, as the temperature rises, the Si atoms free some electrons and generate some holes. But, the number of these holes is very small. Hence, at any given point in time, the number of free electrons is much higher than the number of holes. Also, due to recombination, the number of holes reduce further.
In a nutshell, when a semiconductor is doped with a pentavalent atom, electrons are the majority charge carriers. On the other hand, the holes are the minority charge carriers. Therefore, such extrinsic semiconductors are called n-type semiconductors. In an n-type semiconductor,
Number of free electrons (ne) >> Number of holes (nh)
A p-type semiconductor is created when trivalent elements are used to dope pure semiconductors, like Si and Ge. As can be seen in the image above, when a trivalent atom takes the place of a Si atom, three of its electrons bond with three neighbouring Si atoms. However, there is no electron to bond with the fourth Si atom.
This leads to a hole or a vacancy between the trivalent and the fourth silicon atom. This hole initiates a jump of an electron from the outer orbit of the atom in the neighbourhood to fill the vacancy. This creates a hole at the site from where the electron jumps. In simple words, a hole is now available for conduction.
It is important to remember that the number of holes made available by the dopant atoms is independent of the ambient temperature and primarily depends on the doping level. Also, as the temperature rises, the Si atoms free some electrons and generate some holes. But, the number of these electrons is very small. Hence, at any given point in time, the number of holes is much higher than the number of free electrons. Also, due to recombination, the number of free electrons reduce further.
In a nutshell, when a semiconductor is doped with a trivalent atom, holes are the majority charge carriers. On the other hand, the free electrons are the minority charge carriers. Therefore, such extrinsic semiconductors are called p-type semiconductors. In a p-type semiconductor,
Number of holes (nh) >> Number of free electrons (ne)
Important note: The crystal maintains an overall charge neutrality. The charge of additional charge carries is equal and opposite to that of the ionized cores in the lattice.
In extrinsic semiconductors, a change in the ambient temperature leads to the production of minority charge carriers. Also, the dopant atoms produce the majority carriers. During recombination, the majority carriers destroy most of these minority carriers. This leads to a decrease in the concentration of the minority carriers.
Therefore, this affects the energy band structure of the semiconductor. In such semiconductors, additional energy states exist:
· Energy state due to donor impurity (ED)
· Energy state due to acceptor impurity (EA)
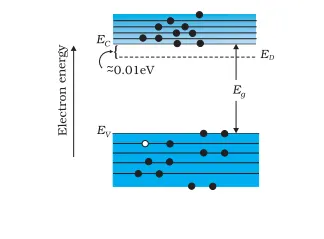
The above energy band diagram is of n-type Si semiconductor. Here you can see that the energy level of the donor (ED) is lower than that of the conduction band (EC). Hence, electrons can move into the conduction band with minimal energy (~0.01 eV). Also, at room temperature, most donor atoms and very few Si atoms get ionized. Hence, the conduction band has most electrons from the donor impurities.
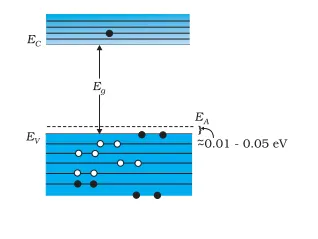
The above energy band diagram is of p-type Si semiconductor. Here you can see that the energy level of the acceptor (EA) is higher than that of the valence band (EV). Hence, electrons can move from the valence band to the level Ea, with minimal energy. Also, at room temperature, most acceptor atoms are ionized.
This leaves holes in the valence band. Hence, the valence band has most holes from the impurities. The electron and hole concentration in a semiconductor in thermal equilibrium is:
ne × nh = ni2